Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement - PubMed (original) (raw)
Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement
Masaaki Yoshigi et al. J Cell Biol. 2005.
Abstract
Organs and tissues adapt to acute or chronic mechanical stress by remodeling their actin cytoskeletons. Cells that are stimulated by cyclic stretch or shear stress in vitro undergo bimodal cytoskeletal responses that include rapid reinforcement and gradual reorientation of actin stress fibers; however, the mechanism by which cells respond to mechanical cues has been obscure. We report that the application of either unidirectional cyclic stretch or shear stress to cells results in robust mobilization of zyxin from focal adhesions to actin filaments, whereas many other focal adhesion proteins and zyxin family members remain at focal adhesions. Mechanical stress also induces the rapid zyxin-dependent mobilization of vasodilator-stimulated phosphoprotein from focal adhesions to actin filaments. Thickening of actin stress fibers reflects a cellular adaptation to mechanical stress; this cytoskeletal reinforcement coincides with zyxin mobilization and is abrogated in zyxin-null cells. Our findings identify zyxin as a mechanosensitive protein and provide mechanistic insight into how cells respond to mechanical cues.
Figures
Figure 1.
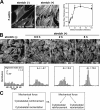
Uniaxial cyclic stretch induces bimodal actin cytoskeletal remodeling: actin stress fiber reinforcement and reorientation. (A) Phalloidin staining of mouse fibroblasts on unstretched membranes (−) or membranes exposed to 1 h of cyclic stretch (+; 15% at 0.5 Hz). The stress fiber thickness index (SFTI) analysis showed actin thickening in a stretch duration–dependent manner (*, P < 0.05). Error bars represent SEM. (B) Labeling of F-actin in fibroblasts exposed to unidirectional cyclic stretch. Quantitative analysis illustrated progressive alignment of the actin cytoskeleton, which was perpendicular to the stretch axis, as a function of stretch durations. (C) Mechanical force induces both cytoskeletal reinforcement and reorientation. These two responses may result from sequential or parallel pathways that are mechanistically related or distinct.
Figure 2.
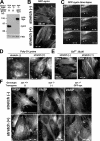
Cyclic stretch induces zyxin mobilization from focal adhesions to the actin cytoskeleton. (A) Fibroblasts on unstretched membranes (−) or subjected to uniaxial cyclic stretch (+; 1 h at 15% and 0.5 Hz) aligned and reinforced their actin filaments (phalloidin staining), whereas the focal adhesion protein vinculin remained at adhesion sites. (B) In contrast to vinculin, unidirectional cyclic stretch resulted in the mobilization of zyxin from focal adhesions to actin filaments. (C) Double labeling of vinculin and zyxin revealed their colocalization at focal adhesions in unstretched cells. Detection of vinculin in focal adhesions of stretched cells in which zyxin has been mobilized to actin filaments clearly illustrates that focal adhesions persist after stretch and highlight the reduced zyxin levels at those sites.
Figure 3.
Stretch-induced kinetics of GFP-zyxin, effects of inhibitors, and VASP localization. (A) Immunoblot analysis with antizyxin antibody in wild-type, zyxin −/−, and zyxin −/− cells expressing full-length GFP-tagged zyxin (GFP-zyx). GFP-zyxin was expressed at a level that was comparable to wild-type cells. FAK immunoblot illustrates equal protein loading. (B) Direct visualization of GFP-zyxin in unstretched and stretched cells. (C) Kinetics of zyxin mobilization to stress fibers in response to stretch. By using a live cell stretching system, GFP-zyxin signals were recorded during 150 s of cyclic stretching. Arrowheads indicate GFP-zyxin accumulation along actin filaments. (D) Cells were allowed to spread on poly-
d
-lysine–coated silicone for 3 h, stretched for 60 min, and immunostained to detect zyxin. (E) Cells were stretched in the presence of Gd3+, and zyxin was localized by immunostaining. (F) Wild-type (+/+), zyxin-null (−/−), and zyxin-null cells expressing GFP-zyxin were stimulated by cyclic stretch (+; 1 h at 15% and 0.5 Hz) and were immunostained to detect VASP.
Figure 4.
Fluid shear stress leads to actin reinforcement, realignment, and mobilization of zyxin and VASP to actin stress fibers. Vascular endothelial cells that were subjected to fluid shear stress (2 h) aligned their actin filaments parallel to the flow direction (arrow). Zyxin and VASP mobilized to actin filaments, whereas vinculin remained at focal adhesions.
Figure 5.
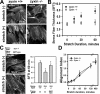
Zyxin is required for actin stress fiber reinforcement in response to mechanical stimulation. (A) F-actin (phalloidin) in zyxin +/+ and −/− fibroblasts before (−) or after (+) cyclic stretch. Note the thinner stress fibers in stretched zyxin −/− cells. (B) SFTI was measured over a 1-h time course of stretch stimulation. Zyxin-null (−/−) fibroblasts showed an impaired actin thickening response (*, P < 0.05). (C) Phalloidin staining and SFTI analysis indicated that the expression of GFP-zyxin in zyxin −/− cells rescued the stress fiber reinforcement response (*, P < 0.05 vs. +/+; †, P < 0.05 vs. −/−). (D) Comparison of the alignment index of zyxin +/+ and −/− cells over an 8-h time course of stretch stimulation. Error bars represent SEM.
Similar articles
- Mechanical signals activate p38 MAPK pathway-dependent reinforcement of actin via mechanosensitive HspB1.
Hoffman L, Jensen CC, Yoshigi M, Beckerle M. Hoffman L, et al. Mol Biol Cell. 2017 Oct 1;28(20):2661-2675. doi: 10.1091/mbc.E17-02-0087. Epub 2017 Aug 2. Mol Biol Cell. 2017. PMID: 28768826 Free PMC article. - Zyxin is not colocalized with vasodilator-stimulated phosphoprotein (VASP) at lamellipodial tips and exhibits different dynamics to vinculin, paxillin, and VASP in focal adhesions.
Rottner K, Krause M, Gimona M, Small JV, Wehland J. Rottner K, et al. Mol Biol Cell. 2001 Oct;12(10):3103-13. doi: 10.1091/mbc.12.10.3103. Mol Biol Cell. 2001. PMID: 11598195 Free PMC article. - Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells.
Schratt G, Philippar U, Berger J, Schwarz H, Heidenreich O, Nordheim A. Schratt G, et al. J Cell Biol. 2002 Feb 18;156(4):737-50. doi: 10.1083/jcb.200106008. Epub 2002 Feb 11. J Cell Biol. 2002. PMID: 11839767 Free PMC article. - Molecular mechanisms underlying the force-dependent regulation of actin-to-ECM linkage at the focal adhesions.
Hirata H, Sokabe M, Lim CT. Hirata H, et al. Prog Mol Biol Transl Sci. 2014;126:135-54. doi: 10.1016/B978-0-12-394624-9.00006-3. Prog Mol Biol Transl Sci. 2014. PMID: 25081617 Review. - Zyxin: a mechanotransductor to regulate gene expression.
Wang YX, Wang DY, Guo YC, Guo J. Wang YX, et al. Eur Rev Med Pharmacol Sci. 2019 Jan;23(1):413-425. doi: 10.26355/eurrev_201901_16790. Eur Rev Med Pharmacol Sci. 2019. PMID: 30657586 Review.
Cited by
- Zyxin is involved in regulation of mechanotransduction in arteriole smooth muscle cells.
Sun Z, Huang S, Li Z, Meininger GA. Sun Z, et al. Front Physiol. 2012 Dec 20;3:472. doi: 10.3389/fphys.2012.00472. eCollection 2012. Front Physiol. 2012. PMID: 23267329 Free PMC article. - CDK1-cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry.
Chen NP, Aretz J, Fässler R. Chen NP, et al. Nat Cell Biol. 2022 May;24(5):723-736. doi: 10.1038/s41556-022-00886-z. Epub 2022 Apr 25. Nat Cell Biol. 2022. PMID: 35469017 Free PMC article. - Direct detection of cellular adaptation to local cyclic stretching at the single cell level by atomic force microscopy.
Watanabe-Nakayama T, Machida SI, Harada I, Sekiguchi H, Afrin R, Ikai A. Watanabe-Nakayama T, et al. Biophys J. 2011 Feb 2;100(3):564-572. doi: 10.1016/j.bpj.2010.12.3693. Biophys J. 2011. PMID: 21281570 Free PMC article. - Mechanical stress triggers nuclear remodeling and the formation of transmembrane actin nuclear lines with associated nuclear pore complexes.
Hoffman LM, Smith MA, Jensen CC, Yoshigi M, Blankman E, Ullman KS, Beckerle MC. Hoffman LM, et al. Mol Biol Cell. 2020 Jul 21;31(16):1774-1787. doi: 10.1091/mbc.E19-01-0027. Epub 2020 Jan 22. Mol Biol Cell. 2020. PMID: 31967947 Free PMC article. - Spatial organization of cell-adhesive ligands for advanced cell culture.
Ekerdt BL, Segalman RA, Schaffer DV. Ekerdt BL, et al. Biotechnol J. 2013 Dec;8(12):1411-23. doi: 10.1002/biot.201300302. Epub 2013 Nov 6. Biotechnol J. 2013. PMID: 24318636 Free PMC article. Review.
References
- Albinsson, S., I. Nordstrom, and P. Hellstrand. 2004. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J. Biol. Chem. 279:34849–34855. - PubMed
- Beckerle, M.C. 1997. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 19:949–957. - PubMed
- Beckerle, M.C. 1998. Spatial control of actin filament assembly: lessons from Listeria. Cell. 95:741–748. - PubMed
- Bershadsky, A.D., N.Q. Balaban, and B. Geiger. 2003. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19:677–695. - PubMed
- Bockholt, S.M., and K. Burridge. 1995. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes. Commun. 3:91–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous