Organotypic epithelial raft cultures as a model for evaluating compounds against alphaherpesviruses - PubMed (original) (raw)
Organotypic epithelial raft cultures as a model for evaluating compounds against alphaherpesviruses
Graciela Andrei et al. Antimicrob Agents Chemother. 2005 Nov.
Abstract
The course of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) and varicella-zoster virus (VZV) infections in squamous epithelial cells cultured in a three-dimensional organotypic raft culture was tested. In these raft cultures, normal human keratinocytes isolated from neonatal foreskins grown at the air-liquid interface stratified and differentiated, reproducing a fully differentiated epithelium. Typical cytopathic changes identical to those found in the squamous epithelium in vivo, including ballooning and reticular degeneration with the formation of multinucleate cells, were observed throughout the raft following infection with HSV and VZV at different times after lifting the cultures to the air-liquid interface. For VZV, the aspects of the lesions depended on the stage of differentiation of the organotypic cultures. The activity of reference antiviral agents, acyclovir (ACV), penciclovir (PCV), brivudin (BVDU), foscarnet (PFA), and cidofovir (CDV), was evaluated against wild-type and thymidine kinase (TK) mutants of HSV and VZV in the raft cultures. ACV, PCV, and BVDU protected the epithelium against cytopathic effect induced by wild-type viruses in a concentration-dependent manner, while treatment with CDV and PFA proved protective against the cytodestructive effects induced by both TK+ and TK- strains. The quantification of the antiviral effects in the rafts were accomplished by measuring viral titers by plaque assay for HSV and by measuring viral DNA load by real-time PCR for VZV. A correlation between the degree of protection as determined by histological examination and viral quantification could be demonstrated The three-dimensional epithelial raft culture represents a novel model for the study of antiviral agents active against HSV and VZV. Since no animal model is available for the evaluation of antiviral agents against VZV, the organotypic cultures may be considered a model to evaluate the efficacy of new anti-VZV antivirals before clinical trials.
Figures
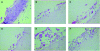
FIG. 1.
Pattern of HSV-1 (KOS strain) infection in cultures infected at day 0 (B), or 2 days (C), 4 days (D), 6 days (E), or 8 days (F) after lifting compared to noninfected cultures (A), where the stratum corneum (a), the well-differentiated epithelium (b) and the collagen matrix with the feeder cells (c) can be seen. Magnification 40.
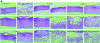
FIG. 2.
Pattern of VZV (OKA and 07-1 strains) infection in cultures infected at day 0 (A), or 2 days (B) 4 days (C), 6 days (D), or 8 days (E) after lifting. Magnification ×40.
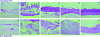
FIG. 3.
Effects of ACV (A) and PCV (B) on organotypic epithelial rafts cultures infected with HSV-1 (KOS strain) at 8 days after lifting. Compounds were added to the culture medium on the day of infection and remained in contact with the cells until the rafts were fixed (i.e., at 12 days after lifting).
FIG. 3.
Effects of ACV (A) and PCV (B) on organotypic epithelial rafts cultures infected with HSV-1 (KOS strain) at 8 days after lifting. Compounds were added to the culture medium on the day of infection and remained in contact with the cells until the rafts were fixed (i.e., at 12 days after lifting).
FIG. 4.
Quantification of virus yield in organotypic epithelial raft cultures infected with HSV-1 and HSV-2 TK+ and TK− strains at 8 days postlifting. Compounds were added to the culture medium the day of infection (i.e., 8 days postdifferentiation) and remained in contact with the cells till the rafts were frozen (i.e., at 12 days after lifting) for determination of virus production by plaque assay.
FIG. 5.
Effects of different antiviral compounds on organotypic epithelial raft cultures infected with 07-1 TK− strain at 5 days after lifting. Compounds were added to the culture medium the day of infection and remained in contact with the cells until the rafts were fixed (i.e., at 12 days after lifting).
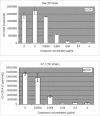
FIG. 6.
Quantification of VZV-DNA by real-time PCR in organotypic epithelial raft cultures infected with Oka (TK+) or 07-1 (TK−). Cidofovir was added to the culture medium the day of infection (5 days postdifferentiation) and remained in contact with the cells till the epithelium was harvested and DNA isolated (i.e., at 12 days after lifting).
Similar articles
- Epithelial raft cultures for investigations of virus growth, pathogenesis and efficacy of antiviral agents.
Andrei G, Duraffour S, Van den Oord J, Snoeck R. Andrei G, et al. Antiviral Res. 2010 Mar;85(3):431-49. doi: 10.1016/j.antiviral.2009.10.019. Epub 2009 Oct 31. Antiviral Res. 2010. PMID: 19883696 Review. - In vitro selection of drug-resistant varicella-zoster virus (VZV) mutants (OKA strain): differences between acyclovir and penciclovir?
Andrei G, De Clercq E, Snoeck R. Andrei G, et al. Antiviral Res. 2004 Mar;61(3):181-7. doi: 10.1016/j.antiviral.2003.10.003. Antiviral Res. 2004. PMID: 15168799 - Antiviral activity of cyclosaligenyl prodrugs of the nucleoside analogue bromovinyldeoxyuridine against herpes viruses.
Meerbach A, Meier C, Sauerbrei A, Meckel HM, Wutzler P. Meerbach A, et al. Int J Antimicrob Agents. 2006 May;27(5):423-30. doi: 10.1016/j.ijantimicag.2005.11.021. Epub 2006 Apr 18. Int J Antimicrob Agents. 2006. PMID: 16621459 - Acyclic/carbocyclic guanosine analogues as anti-herpesvirus agents.
De Clercq E, Andrei G, Snoeck R, De Bolle L, Naesens L, Degrève B, Balzarini J, Zhang Y, Schols D, Leyssen P, Ying C, Neyts J. De Clercq E, et al. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):271-85. doi: 10.1081/NCN-100002298. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563039 Review.
Cited by
- A Novel In Vitro Culture Model System to Study Merkel Cell Polyomavirus-Associated MCC Using Three-Dimensional Organotypic Raft Equivalents of Human Skin.
Loke ASW, Longley BJ, Lambert PF, Spurgeon ME. Loke ASW, et al. Viruses. 2021 Jan 19;13(1):138. doi: 10.3390/v13010138. Viruses. 2021. PMID: 33478104 Free PMC article. - 3D Cell Culture Models in COVID-19 Times: A Review of 3D Technologies to Understand and Accelerate Therapeutic Drug Discovery.
de Dios-Figueroa GT, Aguilera-Marquez JDR, Camacho-Villegas TA, Lugo-Fabres PH. de Dios-Figueroa GT, et al. Biomedicines. 2021 May 26;9(6):602. doi: 10.3390/biomedicines9060602. Biomedicines. 2021. PMID: 34073231 Free PMC article. Review. - Three-dimensional cell culture models for investigating human viruses.
He B, Chen G, Zeng Y. He B, et al. Virol Sin. 2016 Oct;31(5):363-379. doi: 10.1007/s12250-016-3889-z. Epub 2016 Oct 27. Virol Sin. 2016. PMID: 27822716 Free PMC article. Review. - Isolation of Biopsy-Derived, Human Cervical Keratinocytes Propagated as Monolayer and Organoid Cultures.
Villa PL, Jackson R, Eade S, Escott N, Zehbe I. Villa PL, et al. Sci Rep. 2018 Dec 14;8(1):17869. doi: 10.1038/s41598-018-36150-4. Sci Rep. 2018. PMID: 30552408 Free PMC article. - RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation.
Jones M, Dry IR, Frampton D, Singh M, Kanda RK, Yee MB, Kellam P, Hollinshead M, Kinchington PR, O'Toole EA, Breuer J. Jones M, et al. PLoS Pathog. 2014 Jan 30;10(1):e1003896. doi: 10.1371/journal.ppat.1003896. eCollection 2014 Jan. PLoS Pathog. 2014. PMID: 24497829 Free PMC article. Clinical Trial.
References
- Andrei, G., R. Snoeck, and E. De Clercq. 1994. Human brain tumor cell lines as cell substrates to demonstrate sensitivity/resistance of herpes simplex virus types 1 and 2 to acyclic nucleoside analogues. Antiviral Chem. Chemother. 5:263-270.
- Andrei, G., R. Snoeck, E. De Clercq, R. Esnouf, P. Fiten, and G. Opdenakker. 2000. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 81:639-648. - PubMed
- Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources