SegH and Hef: two novel homing endonucleases whose genes replace the mobC and mobE genes in several T4-related phages - PubMed (original) (raw)
SegH and Hef: two novel homing endonucleases whose genes replace the mobC and mobE genes in several T4-related phages
Linus Sandegren et al. Nucleic Acids Res. 2005.
Abstract
T4 contains two groups of genes with similarity to homing endonucleases, the seg-genes (similarity to endonucleases encoded by group I introns) containing GIY-YIG motifs and the mob-genes (similarity to mobile endonucleases) containing H-N-H motifs. The four seg-genes characterized to date encode homing endonucleases with cleavage sites close to their respective gene loci while none of the mob-genes have been shown to cleave DNA. Of 18 phages screened, only T4 was found to have mobC while mobE genes were found in five additional phages. Interestingly, three phages encoded a seg-like gene (hereby called segH) with a GIY-YIG motif in place of mobC. An additional phage has an unrelated gene called hef (homing endonuclease-like function) in place of the mobE gene. The gene products of both novel genes displayed homing endonuclease activity with cleavage site specificity close to their respective genes. In contrast to intron encoded homing endonucleases, both SegH and Hef can cleave their own DNA as well as DNA from phages without the genes. Both segH and mobE (and most likely hef) can home between phages in mixed infections. We discuss why it might be a selective advantage for phage freestanding homing endonucleases to cleave both HEG-containing and HEG-less genomes.
Figures
Figure 1
(A) Alignment of T4 Seg-proteins and LZ2 SegH. (B) Alignment of the different MobE proteins. The sequence of the MobE protein from Schizo-T-even phage AehI was obtained from the genome sequence, GenBank accession no. AY266303. Residues with black shading and white text are identical in 70% of the sequences, grey shading and black text denotes positions with similar residues in 70% of the sequences. (C) Alignment of a region of Hef (residues 251–345) with a region of a hypothetical protein from Corynebacterium glutamicum (residues 283–370 of 606, GenBank accession no. NP_600985) and a region from a representative ORF with unknown function that appears to be related to a diverse group of endonucleases (residues 9–102 of 144 from Rhodopseudomonas palustris hypothetical protein, GenBank accession no. NP_946397). Residues with black shading are conserved, and asterisks denote highly conserved residues among the diverse group of endonucleases.
Figure 2
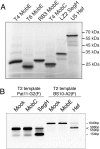
(A) PAGE gel 8% of the in vitro translation products. Proteins were labelled with [35S]Methionine during in vitro translation and bands were visualized by phosphoimager analysis. (B) Agarose gel from cleavage assay with in vitro translated T4 MobC (lane 2), LZ2 SegH (lane 3), T4 MobE (lane 5) and U5 Hef (lane 6); control incubations with in vitro translation mix without template DNA (lanes 1, 4). DNA targets were labelled at one end with Flourescein-labelled primer thereby generating only one labelled band upon cleavage.
Figure 3
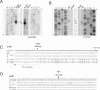
Sequencing reactions run alongside single strand labelled cleavage reactions to map (A) the SegH cleavage site, and (B) the Hef cleavage site. Note that the second gel is inverted to illustrate the cleavage sites on both strands. Sequence variation surrounding (C) the SegH cleavage site in some T-even-like phages with or without optional genes mobC or segH, and (D) the Hef cleavage site in T-even-like phages with and without optional genes mobE or hef.
Figure 4
Competition between target sites for SegH and Hef endonucleases. (A) Purified SegH cleavage of target sites from T2 (triangles), T4 (squares) and LZ2 (circles). (B) Cleavage of T2 (triangles), T4 (squares) and U5 (circles) target sites by in vitro translated Hef. Open symbols denote incubations with twice as much endonuclease as in incubations denoted with closed symbols.
Figure 5
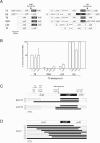
(A) Distribution of optional genes and gene order of flanking genes for the _nrdD_-nrdG, and the _td_-_nrdA_-nrdB regions in phages used as donors and recipients in the homing experiments. Genes are indicated by large boxes, introns with thin boxes and intergenic regions with black lines. Optional genes are dark grey with white text. Note that frame-shifted HEGs are indicated by short/interrupted ORF boxes. The cleavage positions of SegH and Hef are shown. (B) Frequency of screened introns and HEGs in progeny of mixed infections. Error bars show the standard error of the mean. Homing proficient genes are generally over represented in progeny from mixed infections (i.e. U5 td IVS) while non-homing genes are expected to have around 50% occurrence (i.e. U5 nrdD IVS). General exclusion by the recipient phage can reduce the effect of homing and therefore some homing proficient genes are present in less than 50% of the progeny. Such progeny were screened for recombination points between donor and recipient sequences close to the gene of interest, indicative of homing. (C) Screen for co-conversion of flanking markers around the segH gene in RB3/T2 and LZ2/T2 crosses. (D) Screen for co-conversion of flanking markers around the mobE in T6/T2 crosses. Gene order and position of restriction sites used to determine sequence specific markers are shown at the top. White boxes denote recipient alleles, black boxes denote donor alleles and dashed lines denote regions where recombination has occurred. Frequencies of the different chimerical sequences in the progeny are indicated to the left.
Figure 6
(A) Proposed pathway for transposition of a HEG via cleavage of its own genome. Occasional cleavage of the own genome by the homing endonuclease at the cleavage site gives it the opportunity to invade sites with sequence similarity at other positions in the genome, or in another genome during a mixed infection event. Single strand invasion followed by Join-cut-copy replication (38) and resolution of the Holiday junction will form a chimeric chromosome where the HEG has been joined to the new site. Short regions of initial pairing will be stabilized by DNA replication from the 3′ end of the invading strand. In those cases where the homing endonuclease can cleave the alternative site the chimeric chromosome may subsequently be used as template for double strand break repair (DSBR) using the identical part (black lines) and any other short sequence similarity on the opposite side of the HEG for initiation of repair. This will result in the HEG being inserted at the new position in the genome. (B) Scheme of horizontal transfer of HEG to a cognate site within a phage population and invasion and spread to new sites within the same genome or an unrelated genome. The scheme is an adaptation of the cycle of intron gain and loss from reference (19). Heavy arrows indicate preferred transfer, and light arrows less frequent events.
Figure 6
(A) Proposed pathway for transposition of a HEG via cleavage of its own genome. Occasional cleavage of the own genome by the homing endonuclease at the cleavage site gives it the opportunity to invade sites with sequence similarity at other positions in the genome, or in another genome during a mixed infection event. Single strand invasion followed by Join-cut-copy replication (38) and resolution of the Holiday junction will form a chimeric chromosome where the HEG has been joined to the new site. Short regions of initial pairing will be stabilized by DNA replication from the 3′ end of the invading strand. In those cases where the homing endonuclease can cleave the alternative site the chimeric chromosome may subsequently be used as template for double strand break repair (DSBR) using the identical part (black lines) and any other short sequence similarity on the opposite side of the HEG for initiation of repair. This will result in the HEG being inserted at the new position in the genome. (B) Scheme of horizontal transfer of HEG to a cognate site within a phage population and invasion and spread to new sites within the same genome or an unrelated genome. The scheme is an adaptation of the cycle of intron gain and loss from reference (19). Heavy arrows indicate preferred transfer, and light arrows less frequent events.
Similar articles
- Phage T4 mobE promotes trans homing of the defunct homing endonuclease I-TevIII.
Wilson GW, Edgell DR. Wilson GW, et al. Nucleic Acids Res. 2009 Nov;37(21):7110-23. doi: 10.1093/nar/gkp769. Nucleic Acids Res. 2009. PMID: 19773422 Free PMC article. - Genetic insertions and diversification of the PolB-type DNA polymerase (gp43) of T4-related phages.
Petrov VM, Ratnayaka S, Karam JD. Petrov VM, et al. J Mol Biol. 2010 Jan 22;395(3):457-74. doi: 10.1016/j.jmb.2009.10.054. Epub 2009 Nov 5. J Mol Biol. 2010. PMID: 19896487 - DNA binding and cleavage by the nuclear intron-encoded homing endonuclease I-PpoI.
Flick KE, Jurica MS, Monnat RJ Jr, Stoddard BL. Flick KE, et al. Nature. 1998 Jul 2;394(6688):96-101. doi: 10.1038/27952. Nature. 1998. PMID: 9665136 - Homing endonuclease structure and function.
Stoddard BL. Stoddard BL. Q Rev Biophys. 2005 Feb;38(1):49-95. doi: 10.1017/S0033583505004063. Epub 2005 Dec 9. Q Rev Biophys. 2005. PMID: 16336743 Review. - Mobile DNA elements in T4 and related phages.
Edgell DR, Gibb EA, Belfort M. Edgell DR, et al. Virol J. 2010 Oct 28;7:290. doi: 10.1186/1743-422X-7-290. Virol J. 2010. PMID: 21029434 Free PMC article. Review.
Cited by
- Amino acid residues in the GIY-YIG endonuclease II of phage T4 affecting sequence recognition and binding as well as catalysis.
Lagerbäck P, Carlson K. Lagerbäck P, et al. J Bacteriol. 2008 Aug;190(16):5533-44. doi: 10.1128/JB.00094-08. Epub 2008 Jun 6. J Bacteriol. 2008. PMID: 18539732 Free PMC article. - Nuclease genes occupy boundaries of genetic exchange between bacteriophages.
Barth ZK, Dunham DT, Seed KD. Barth ZK, et al. NAR Genom Bioinform. 2023 Aug 24;5(3):lqad076. doi: 10.1093/nargab/lqad076. eCollection 2023 Sep. NAR Genom Bioinform. 2023. PMID: 37636022 Free PMC article. - A functional homing endonuclease in the Bacillus anthracis nrdE group I intron.
Nord D, Torrents E, Sjöberg BM. Nord D, et al. J Bacteriol. 2007 Jul;189(14):5293-301. doi: 10.1128/JB.00234-07. Epub 2007 May 11. J Bacteriol. 2007. PMID: 17496101 Free PMC article. - Insertion of a homing endonuclease creates a genes-in-pieces ribonucleotide reductase that retains function.
Friedrich NC, Torrents E, Gibb EA, Sahlin M, Sjöberg BM, Edgell DR. Friedrich NC, et al. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6176-81. doi: 10.1073/pnas.0609915104. Epub 2007 Mar 29. Proc Natl Acad Sci U S A. 2007. PMID: 17395719 Free PMC article. - Phage T4 mobE promotes trans homing of the defunct homing endonuclease I-TevIII.
Wilson GW, Edgell DR. Wilson GW, et al. Nucleic Acids Res. 2009 Nov;37(21):7110-23. doi: 10.1093/nar/gkp769. Nucleic Acids Res. 2009. PMID: 19773422 Free PMC article.
References
- Dujon B. Group I introns as mobile genetic elements: facts and mechanistic speculations—a review. Gene. 1989;82:91–114. - PubMed
- Belfort M., Perlman P.S. Mechanisms of intron mobility. J. Biol. Chem. 1995;270:30237–30240. - PubMed
- Perlman P.S., Butow R.A. Mobile introns and intron-encoded proteins. Science. 1989;246:1106–1109. - PubMed
- Lambowitz A.M. Infectious introns. Cell. 1989;56:323–326. - PubMed
- Belfort M. Phage T4 introns: self-splicing and mobility. Annu. Rev. Genet. 1990;24:363–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases