Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia - PubMed (original) (raw)
Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia
Ami A Deora et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Proton-coupled monocarboxylate transporters (MCT) MCT1, MCT3, and MCT4 form heterodimeric complexes with the cell surface glycoprotein CD147 and exhibit tissue-specific polarized distributions that are essential for maintaining lactate and pH homeostasis. In the parenchymal epithelia of kidney, thyroid, and liver, MCT/CD147 heterocomplexes are localized in the basolateral membrane where they transport lactate out of or into the cell depending on metabolic conditions. A unique distribution of lactate transporters is found in the retinal pigment epithelium (RPE), which regulates lactate levels of the outer retina. In RPE, MCT1/CD147 is polarized to the apical membrane and MCT3/CD147 to the basolateral membrane. The mechanisms responsible for tissue-specific polarized distribution of MCTs are unknown. Here, we demonstrate that CD147 carries sorting information for polarized targeting of the MCT1/CD147 hetero-complexes in kidney and RPE cells. In contrast, MCT3 and MCT4 harbor dominant sorting information that cotargets CD147 to the basolateral membrane in both epithelia. RNA interference experiments show that MCT1 promotes CD147 maturation. Our results open a unique paradigm to study the molecular basis of tissue-specific polarity.
Figures
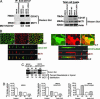
Fig. 1.
Transfected rat CD147-L252A redirects endogenous MCT1 to the apical surface of MDCK cells. (A) Coimmunoprecipitation of CD147 and MCT1. The interaction between CD147 and MCT1 was studied by immunoprecipitation in MDCK cells stably transfected with CD147-WT-GFP or CD147-L252A-GFP, using an Ab against rat CD147 (RET-PE2). We used GFP-tagged CD147 for coimmunoprecipitation so that the Ab heavy chain would not mask the untagged 50-kDa CD147 band. Our previous studies have shown that a C-terminal GFP tag does not affect the trafficking of CD147 (29). The immunoprecipitate (Left) and the cell lysate (Right) were resolved by SDS/PAGE and Western blotted with CD147, MCT1, and β-actin Abs. The amounts of MCT1 interacting with either CD147-WT or CD147-L252A are similar. (B) LSCM analysis of clones of MDCK cells stably overexpressing CD147-WT (Left) or the apically targeted mutant CD147-L252A (Right). Shown are x-y and x-z projections of monolayers of MDCK cells immunostained for CD147-WT or CD147-L252A (green) and of endogenous MCT1 (red). Note that cells expressing CD147-L252A target endogenous MCT1 to the apical membrane; cells that expressed low or null levels of CD147-L252A targeted MCT1 basolaterally (Right; x-z section, arrow). (Scale bar, 10 μm.) (C) Domain-specific cell surface biotinylation was performed on MDCK cells expressing CD147-WT or CD147-L252A. After streptavidin-agarose precipitation, samples were analyzed by SDS/PAGE and Western blot. The results confirm that MDCK cells expressing CD147-L252A targets most of the (86%) endogenous MCT1 to the apical domain. (D) 14C-lactate uptake was performed for 1 min in monolayers confluent on Transwells. WT MDCK and MDCK cells expressing CD147-WT exhibited two times higher lactate uptake on the basolateral side than on the apical side. In contrast, MDCK cells expressing CD147-L252A had a 14C-lactate uptake 1.6-fold higher from the apical side. Hence, apically targeted MCT1 was functional. The MCT inhibitor α-cyano-4-hydroxycinnamate inhibited uptake by 60-70%. Data shown represent mean ± SD (n = 6). BL, basolateral; AP, apical.
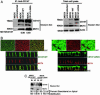
Fig. 2.
CD147 does not control the polarized distribution of MCT4/CD147 complex. (A) Coimmunoprecipitation of CD147 and MCT4. The interaction of rat CD147-WT-GFP and CD147-L252A-GFP and canine MCT4 in MDCK was studied by coimmunoprecipitation with CD147 Ab and Western blotting in stably transfected MDCK cells. (Right) A Western blot of cell lysates is shown for comparison. (Left) Note that canine MCT4 coimmunoprecipitates with rat CD147-WT and CD147-L252A and the amount of MCT4 interacting with either of them is very similar. (B) LSCM analysis of clonal MDCK cell lines expressing CD147-WT-GFP (green) (Left) or CD147-L252A-GFP (green) (Right) were immunostained for endogenous MCT4 (red). x-y image represents maximum projection of the entire stack. (Right) Note that overexpression of apically targeted CD147-L252A did not affect the basolateral localization of MCT4. However, MCT4 colocalizes with the basolateral pool of CD147-L252A. (Scale bar, 10 μm.) (C) Domain-specific cell surface biotinylation followed by streptavidin-agarose pull down. Note that MCT4 is localized on the basolateral domain irrespective of the CD147 localization. BL, basolateral; AP, apical.
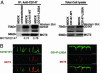
Fig. 3.
CD147 does not regulate the polarized distribution of MCT3/CD147 complex. (A) Coimmunoprecipitation of CD147 and MCT3. MDCK stable clones (CD147-WT and CD147-L252A) were transfected with human MCT3. (Left) Immunoprecipitation with RET-PE2 Ab against rat CD147 followed by Western blot for MCT3 in the immunoprecipitate confirmed that the human MCT3 interacts with the rat CD147-WT and CD147-L252A. (Right) Western blot of total cell lysate. (B) LSCM of CD147 and MCT3. Clonal MDCK cell lines overexpressing GFP-tagged rat CD147 (WT or L252A mutant) were transfected with human MCT3. Shown are LSCM x-z sections of these MDCK cell lines immunostained for MCT3 (red) and CD147-WT (green) (Left) or CD147-L252A (green) (Right). (Right) Note that overexpression of apically targeted CD147-L252A did not affect the basolateral localization of MCT3. Similar to MCT4/CD147 complex, the basolateral pool of CD147-L252A forms complex with MCT3. (Scale bar, 10 μm.)
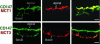
Fig. 4.
Apical MCT1, basolateral MCT3, and predominantly apical CD147 in human fetal RPE. LSCM analysis of cryosection (8 μm) of polarized human fetal RPE cells grown on filters show apical localization of CD147 (green) and MCT1 (red) (Upper) and MCT3 (red) (Lower) colocalizing with basolateral pool of CD147 (green). (Scale bar, 10 μm.)
Fig. 5.
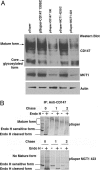
MCT1 is essential for the maturation of CD147. (A) Western blot analysis of RNAi-treated Caco-2 cells. C2BBE, a subline of human Caco-2 cells, expresses both MCT1 and CD147 endogenously. Although these cells also express the MCT4 mRNA, we did not observe expression of MCT4 protein by Western blot (data not shown). CD147 and MCT1 were silenced by using pSuper vector-based siRNA. After transfection (72 h) of various pSuper constructs (pSuper, pSuper CD147130scrambled, pSuper CD147 130, pSuper MCT1 423scrambled, and pSuper MCT1 423), 20 μg of cell lysate was resolved by SDS/PAGE and analyzed by Western blot for the presence and levels of CD147 and MCT1. Protein levels were normalized against actin levels measured by a β-actin Ab. Note that down-regulation of CD147 led to concomitant reduction in the expression of MCT1 protein. Note also that silencing of MCT1 led to maturation arrest of CD147 in its core glycosylated form, demonstrating that MCT1 acts as a chaperone for CD147. (B) Pulse-chase analysis of RNAi-treated Caco cells. C2BBE1 cells transfected with either pSuper or pSuper-MCT1 423 plasmids were pulsed with [35S]Met/Cys for 30 min and chased for 0, 1, and 2 h in the presence of excess cold amino acids. Immunoprecipitates obtained with human CD147 Ab were treated with Endo-H (+) or not (-), and the samples were analyzed by SDS/PAGE and analyzed by PhosphorImager (Molecular Dynamics). Control cells treated with empty pSuper vector show normal maturation of CD147 to Endo-H resistance within 1 h; only a small amount of immature Endo-H-sensitive form is seen at this time. In contrast, silencing of MCT1 hinders maturation of CD147 to Endo-H resistance. Under these conditions, Endo-H-sensitive CD147 is seen even after a 2-h chase.
Similar articles
- Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells.
Philp NJ, Wang D, Yoon H, Hjelmeland LM. Philp NJ, et al. Invest Ophthalmol Vis Sci. 2003 Apr;44(4):1716-21. doi: 10.1167/iovs.02-0287. Invest Ophthalmol Vis Sci. 2003. PMID: 12657613 - Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse.
Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Philp NJ, et al. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):1305-11. doi: 10.1167/iovs.02-0552. Invest Ophthalmol Vis Sci. 2003. PMID: 12601063 - Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.
Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ. Castorino JJ, et al. Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article. - The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation.
Halestrap AP, Price NT. Halestrap AP, et al. Biochem J. 1999 Oct 15;343 Pt 2(Pt 2):281-99. Biochem J. 1999. PMID: 10510291 Free PMC article. Review. - The SLC16 gene family - structure, role and regulation in health and disease.
Halestrap AP. Halestrap AP. Mol Aspects Med. 2013 Apr-Jun;34(2-3):337-49. doi: 10.1016/j.mam.2012.05.003. Mol Aspects Med. 2013. PMID: 23506875 Review.
Cited by
- Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function.
Zhang S, Xu W, Wang H, Cao M, Li M, Zhao J, Hu Y, Wang Y, Li S, Xie Y, Chen G, Liu R, Cheng Y, Xu Z, Zou K, Gong S, Geng L. Zhang S, et al. Cell Prolif. 2019 Nov;52(6):e12673. doi: 10.1111/cpr.12673. Epub 2019 Aug 16. Cell Prolif. 2019. PMID: 31418947 Free PMC article. - Impact of Pals1 on Expression and Localization of Transporters Belonging to the Solute Carrier Family.
Berghaus C, Groh AC, Breljak D, Ciarimboli G, Sabolić I, Pavenstädt H, Weide T. Berghaus C, et al. Front Mol Biosci. 2022 Feb 16;9:792829. doi: 10.3389/fmolb.2022.792829. eCollection 2022. Front Mol Biosci. 2022. PMID: 35252349 Free PMC article. - MCT1 is a predictive marker for lenalidomide maintenance therapy in multiple myeloma.
Stroh J, Seckinger A, Heider M, Rudelius M, Eichner R, Schick M, Slawska J, Emde-Rajaratnam M, Salwender H, Bertsch U, Goldschmidt H, Weisel K, Scheid C, Keller U, Hose D, Bassermann F. Stroh J, et al. Blood Adv. 2022 Jan 25;6(2):515-520. doi: 10.1182/bloodadvances.2021005532. Blood Adv. 2022. PMID: 34768284 Free PMC article. - Generation of functional scFv intrabody to abate the expression of CD147 surface molecule of 293A cells.
Tragoolpua K, Intasai N, Kasinrerk W, Mai S, Yuan Y, Tayapiwatana C. Tragoolpua K, et al. BMC Biotechnol. 2008 Jan 29;8:5. doi: 10.1186/1472-6750-8-5. BMC Biotechnol. 2008. PMID: 18226275 Free PMC article. - Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer.
Pértega-Gomes N, Vizcaíno JR, Miranda-Gonçalves V, Pinheiro C, Silva J, Pereira H, Monteiro P, Henrique RM, Reis RM, Lopes C, Baltazar F. Pértega-Gomes N, et al. BMC Cancer. 2011 Jul 25;11:312. doi: 10.1186/1471-2407-11-312. BMC Cancer. 2011. PMID: 21787388 Free PMC article.
References
- Yeaman, C., Grindstaff, K. K., Hansen, M. D. & Nelson, W. J. (1999) Curr. Biol. 9, R515-R517. - PubMed
- Rodriguez-Boulan, E., Kreitze, G. & Musch, A. (2005) Nat. Rev. Mol. Cell Biol. 6, 233-247. - PubMed
- Keller, P. & Simons, K. (1997) J. Cell Sci. 110, 3001-3009. - PubMed
- Dunbar, L. A. & Caplan, M. J. (2001) J. Biol. Chem. 276, 29617-29620. - PubMed
- Verrey, F., Jack, D. L., Paulsen, I. T., Saier, M. H., Jr., & Pfeiffer, R. (1999) J. Membr. Biol. 172, 181-192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 EY012042/EY/NEI NIH HHS/United States
- R01 GM034107/GM/NIGMS NIH HHS/United States
- R01 EY000444/EY/NEI NIH HHS/United States
- P30 EY000331/EY/NEI NIH HHS/United States
- EY012042/EY/NEI NIH HHS/United States
- EY08538/EY/NEI NIH HHS/United States
- EY00331/EY/NEI NIH HHS/United States
- R01 EY008538/EY/NEI NIH HHS/United States
- Z01 EY000444/Intramural NIH HHS/United States
- GM34107/GM/NIGMS NIH HHS/United States
- R01 EY012042/EY/NEI NIH HHS/United States
- F32 EY014307/EY/NEI NIH HHS/United States
- EY14307/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources