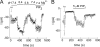Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels - PubMed (original) (raw)
Comparative Study
Charge screening by internal pH and polyvalent cations as a mechanism for activation, inhibition, and rundown of TRPM7/MIC channels
J Ashot Kozak et al. J Gen Physiol. 2005 Nov.
Abstract
The Mg2+-inhibited cation (MIC) current, believed to represent activity of TRPM7 channels, is found in lymphocytes and mast cells, cardiac and smooth muscle, and several other eukaryotic cell types. MIC current is activated during whole-cell dialysis with divalent-free internal solutions. Millimolar concentrations of intracellular Mg2+ (or other divalent metal cations) inhibit the channels in a voltage-independent manner. The nature of divalent inhibition and the mechanism of channel activation in an intact cell remain unknown. We show that the polyamines (spermine, spermidine, and putrescine) inhibit the MIC current, also in a voltage-independent manner, with a potency that parallels the number of charges. Neomycin and poly-lysine also potently inhibited MIC current in the absence of Mg2+. These same positively charged ions inhibited IRK1 current in parallel with MIC current, suggesting that they probably act by screening the head group phosphates on PIP2 and other membrane phospholipids. In agreement with this hypothesis, internal protons also inhibited MIC current. By contrast, tetramethylammonium, tetraethylammonium, and hexamethonium produced voltage-dependent block but no inhibition. We show that inhibition by internal polyvalent cations can be relieved by alkalinizing the cytosol using externally applied ammonium or by increasing pH in inside-out patches. Furthermore, in perforated-patch and cell-attached recordings, when intracellular Mg2+ is not depleted, endogenous MIC or recombinant TRPM7 currents are activated by cytosolic alkalinization and inhibited by acidification; and they can be reactivated by PIP2 following rundown in inside-out patches. We propose that MIC (TRPM7) channels are regulated by a charge screening mechanism and may function as sensors of intracellular pH.
Figures
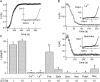
Figure 1.
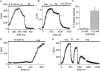
Inhibition of MIC current by internal polyvalent cations and NH4 +. RBL cells with standard internal solution and 2 Ca2+ external solution. All current amplitudes were measured at +85 mV during voltage ramp stimuli. (A) Time course of IMIC development during whole-cell recording and dialysis with 10 mM EDTA. Outward MIC current amplitude is plotted against time after break-in. The inset shows I-V relations obtained at break-in and after 10 min of recording. (B) The time course of inhibition of preactivated MIC current by La3+ (top). The pipette solution contained 1 mM EDTA + 3 mM La3+. The inset shows the I-V relation at break-in and after complete inhibition. The bottom panel shows the time course of inhibition of preactivated MIC current by spermine. The pipette solution contained 10 mM EDTA + 5 mM spermine. The inset shows I-V relations obtained at break-in and after complete inhibition. (C) Maximal current densities with control (1 mM, n = 5 cells) or 10 mM EDTA (n = 3) solutions and with solutions containing inhibitory cations. The cation concentrations in mM were: 5 Ca2+ (n = 5); 3 La3+ (n = 8); 8 putrescine (n = 4); 5 spermidine (n = 5); 4 spermine (n = 5); 6 neomycin (n = 5); 4 polylysine (n = 6); 112 NH4 + (n = 5). *, P < 0.005 compared with controls in 1 or 10 mM EDTA. **, P = 0.16.
Figure 2.
Block of outward MIC current by internal quaternary ammonium derivatives and hexamethonium. Human resting T cells (A–D) and RBL cells (E and F) with 130 mM Cs+ glutamate, 8 mM NaCl, 10 mM EDTA, 0.9 mM CaCl2, 10 mM HEPES, pH 7.3; and 10 HEDTA-Cs+ (A–D) and 1 HEDTA-Cs+ (E and F) external solution. Divalent-free external solution permits inward monovalent current recording. I-V relations were obtained at break-in (red traces) and after full development of the current with internal solutions containing no blocker (A) or 20 mM NH4 + (B), TMA (C), TEA (D), and hexamethonium (E). TMA, TEA, and hexamethonium, but not NH4 +, produced voltage-dependent block of the outward MIC current. (E) Inward MIC current in the presence and absence of 16 mM hexamethonium. The values are not statistically different (P > 0.05).
Figure 3.
Neomycin inhibition of MIC and IRK1 currents. RBL cells with 10 mM EDTA-containing internal solution and 4.5 K+, 2 Ca2+ external solution. (A) Time course of development of MIC (measured at +85 mV) and IRK1 (measured at −110 mV) currents. (B) Neomycin (3 mM) inhibits preactivated MIC and IRK1 currents from inside. (C) I-V relations obtained from B at times 1–3. The plot on top is enlarged below to show that neomycin block precedes its inhibition of IRK1 current. The red trace shows blocked (but not inhibited) IRK1 current. The arrow indicates outward IRK1 current that is rapidly blocked by neomycin diffusing into the cell.
Figure 4.
External NH4 + effect on MIC current in the presence of internal Mg2+. RBL cells in 2 Ca2+ external solution. (A) The internal solution contained 12 mM EGTA and 5 mM MgCl2 ([Mg2+]free = 3 mM). Application of external 81 mM NH4 + reversed inhibition by internal Mg2+ in several successive trials; Mg2+ inhibition was reestablished upon washout of NH4 +. (B) NH4 + prevents inhibition of preactivated MIC current by 2.3 mM free Mg2+ (12 mM EGTA + 4 mM MgCl2).
Figure 5.
Effect of external NH4 + on MIC current in the absence of internal Mg2+: recovery from rundown. RBL cells in 2 Ca2+ external solution with 12 mM EGTA internal solution. (A) MIC current was allowed to develop, and I-V plots obtained at break-in and after the current was fully developed (shown in red color). NH4 + at varying concentrations (20 mM, 81 mM, and 162 mM) was applied subsequently and I-V plots are shown. Note the inward current activated in external NH4 +-containing solution. (B) Rapid application of 81 mM NH4 + causes reversible slow potentiation of the MIC current. (C) MIC current development and rundown. 12 mM EGTA, 100 μM MgCl2 internal solution, [Mg2+]free = 54 μM. 81 mM NH4 + applied after rundown resulted in recovery of IMIC. (D) Dependence of MIC current rundown on Mg2+ and neomycin. Current amplitude (+85 mV) was obtained 30 min (I30 min) after break-in, divided by maximal amplitude (Imax) in the same cell, and the ratio plotted for 12 mM EGTA (free Mg2+ = 54 μM; n = 10 cells), 12 mM EGTA + 0.2 mM EDTA (free Mg2+ = 2 μM, n = 5), 8 mM EGTA + 4 mM EDTA (free Mg2+ = 52 nM, n = 7) and 10 mM EDTA+ 500 μM neomycin (n = 3) internal solutions. *, P < 0.005 compared with the value for 2 μM free Mg2+.
Figure 6.
Effects of external acetate ion on MIC current in RBL cells. (A) MIC current inhibition by application of external acetate when internal solution contained 10 mM HEPES. (B) Increasing internal pH buffering with 122 mM HEPES shifts the acetate effect to higher concentrations. (C) Summary of 40 mM acetate inhibition of MIC current with 10 mM (n = 5) or 122 mM (n = 3) HEPES in the pipette (*, P < 0.005 compared with the value obtained with 10 mM HEPES). The current amplitude was measured at 360 s after the start of acetate application, divided by the amplitude immediately before acetate addition, and plotted as percent of control current. (D) MIC current fails to develop with 0 Mg2+ inside in the presence of external 40 mM acetate. Upon washout of acetate, the current developed normally. (E) Reversible MIC current inhibition by propionate (40 mM) and acetate (40 mM) in the same cell. After prolonged dialysis, the current ran down completely in the absence of external acetate. MIC current amplitudes were measured at + 85 mV.
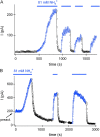
Figure 7.
Activation of MIC current by NH4 + in perforated-patch recording. RBL cell in 2 Ca2+ external solution; amphotericin B–containing pipette solution. (A) MIC current failed to develop during recording before application of external 162 NH4 + which resulted in the development of a robust current. IMIC declined slowly upon removal of NH4 + and rapidly upon addition of 40 mM propionate-containing solution. Both propionate and NH4 + effects were reversible. (B) I-V relations obtained from the experiment shown in A in the presence of external NH4 + and propionate.
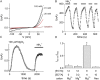
Figure 8.
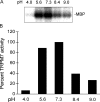
pH dependence of TRPM7 kinase domain phosphotransferase activity. (A) TRPM7 kinase domain phosphorylation of MBP (myelin basic protein) was assessed in vitro at various pH values. The panel shows an autoradiogram of radioactive ATP-labeled MBP after phosphorylation in buffer at various pH values. (B) The kinase activity of TRPM7 was quantified by comparing the intensity of the P32 signal shown in A. Band intensity at 7.3 was set as 100%. The activity was substantially reduced at pH 4.0 and 9. The figure is representative of three separate experiments.
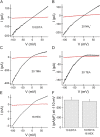
Figure 9.
The pHi dependence of recombinant TRPM7 in whole-cell and inside-out patch recordings. (A) Time course of internal pH inhibition and recovery by external NH4 + during whole-cell recording of overexpressed mTRPM7 in a CHO cell. pH 5.6 internal solution; 2 Ca2+ external solution. Whole-cell recording was initiated at arrow. TRPM7 current amplitude was measured at +85 mV. (B) Individual I-V traces obtained from A. (C) Inside-out patch recording; time or patch excision indicated by arrow. Pipette and bath solutions were 1 mM HEDTA, 154 mM Cs+ aspartate to minimize rundown. Divalent-free external solution permits inward monovalent current recording. pH 5.6 at cytoplasmic surface inhibited TRPM7 fully and reversibly. In the same patch, 2 mM MgCl2 also inhibited the current. (D) Currents at −100 mV and during voltage ramps to +85 mV. (E and F) Increasing the pH from 7.4 to 8.4 increased TRPM7 current amplitude in inside-out patches.
Figure 10.
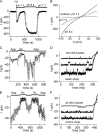
PI(4,5)P2 and pH effects on TRPM7 current in inside-out patch. CHO cell overexpressing mTRPM7. Divalent-free external solution permits inward monovalent current recording. (A) Pipette and bath contained 1 mM HEDTA, 154 mM Cs+ aspartate. pH 8.4 increased current whereas pH 5.6 completely inhibited it. 5 μM PIP2-diC8 application in pH 7.3 mimicked the effect of increased pH. 2 mM MgCl2 was applied. (B) Bath solution contained Cs+ glutamate, 10 EGTA. Establishment of inside-out configuration (i-o) resulted in increased current due to Mg2+ washout followed by rapid rundown. Application of 5 μM PIP2-diC8 to the cytosolic side of the membrane resulted in TRPM7 current recovery.
Figure 11.
TRPM7 current recovery from rundown; inhibition in cell-attached mode. CHO cells overexpressing mTRPM7 WT and TAP mutant. (A and B) Inside-out patch. Monovalent TRPM7 current was allowed to run down fully in 1 mM EGTA, pH 7.3 bath solution. Current recovered and was potentiated by applying pH 8.4 solution to the cytosolic side. Upon return to pH 7.3, the current was inhibited again (compare with Fig. 5 C). (C and D) Cell-attached patch. Sensitivity of ITRPM7 to internal pH changes. The basal ∼40 pS channel activity was inhibited when bath solution was switched from aspartate to acetate in order to acidify the cytosol of the intact cell. The acetate effect was reversible. (E) Sensitivity of ITRPM7-TAP (kinase-dead mutant) to internal pH changes in the cell-attached mode. Constitutive activity of TRPM7-TAP channels was monitored in cell-attached patch in 2 Ca2+ bath solution. Repeated addition of 40 mM acetate to the bath inhibited channel activity. (F) Traces from experiment shown in E. The current trace in the middle represents current before acetate application.
Similar articles
- Polyvalent cations as permeant probes of MIC and TRPM7 pores.
Kerschbaum HH, Kozak JA, Cahalan MD. Kerschbaum HH, et al. Biophys J. 2003 Apr;84(4):2293-305. doi: 10.1016/S0006-3495(03)75035-8. Biophys J. 2003. PMID: 12668438 Free PMC article. - ATP and PIP2 dependence of the magnesium-inhibited, TRPM7-like cation channel in cardiac myocytes.
Gwanyanya A, Sipido KR, Vereecke J, Mubagwa K. Gwanyanya A, et al. Am J Physiol Cell Physiol. 2006 Oct;291(4):C627-35. doi: 10.1152/ajpcell.00074.2006. Epub 2006 May 17. Am J Physiol Cell Physiol. 2006. PMID: 16707555 - Ring of negative charge in BK channels facilitates block by intracellular Mg2+ and polyamines through electrostatics.
Zhang Y, Niu X, Brelidze TI, Magleby KL. Zhang Y, et al. J Gen Physiol. 2006 Aug;128(2):185-202. doi: 10.1085/jgp.200609493. Epub 2006 Jul 17. J Gen Physiol. 2006. PMID: 16847096 Free PMC article. - TRPM7, the Mg(2+) inhibited channel and kinase.
Bates-Withers C, Sah R, Clapham DE. Bates-Withers C, et al. Adv Exp Med Biol. 2011;704:173-83. doi: 10.1007/978-94-007-0265-3_9. Adv Exp Med Biol. 2011. PMID: 21290295 Review. - The Mg2+ and Mg(2+)-nucleotide-regulated channel-kinase TRPM7.
Penner R, Fleig A. Penner R, et al. Handb Exp Pharmacol. 2007;(179):313-28. doi: 10.1007/978-3-540-34891-7_19. Handb Exp Pharmacol. 2007. PMID: 17217066 Free PMC article. Review.
Cited by
- TRPM7, the cytoskeleton and neuronal death.
Asrar S, Aarts M. Asrar S, et al. Channels (Austin). 2013 Jan 1;7(1):6-16. doi: 10.4161/chan.22824. Epub 2012 Dec 17. Channels (Austin). 2013. PMID: 23247582 Free PMC article. Review. - The functional network of ion channels in T lymphocytes.
Cahalan MD, Chandy KG. Cahalan MD, et al. Immunol Rev. 2009 Sep;231(1):59-87. doi: 10.1111/j.1600-065X.2009.00816.x. Immunol Rev. 2009. PMID: 19754890 Free PMC article. Review. - TRPM7-Mediated Calcium Transport in HAT-7 Ameloblasts.
Kádár K, Juhász V, Földes A, Rácz R, Zhang Y, Löchli H, Kató E, Köles L, Steward MC, DenBesten P, Varga G, Zsembery Á. Kádár K, et al. Int J Mol Sci. 2021 Apr 13;22(8):3992. doi: 10.3390/ijms22083992. Int J Mol Sci. 2021. PMID: 33924361 Free PMC article. - The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis.
Nadolni W, Zierler S. Nadolni W, et al. Cells. 2018 Aug 17;7(8):109. doi: 10.3390/cells7080109. Cells. 2018. PMID: 30126133 Free PMC article. Review. - Cell death induction and protection by activation of ubiquitously expressed anion/cation channels. Part 3: the roles and properties of TRPM2 and TRPM7.
Okada Y, Numata T, Sabirov RZ, Kashio M, Merzlyak PG, Sato-Numata K. Okada Y, et al. Front Cell Dev Biol. 2023 Sep 29;11:1246955. doi: 10.3389/fcell.2023.1246955. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37842082 Free PMC article. Review.
References
- Aidley, D.J., and P.R. Stanfield. 1996. Ion channels. Cambridge University Press, Cambridge. 307 pp.
- Bell, J. 1986. The sarcoplasmic reticulum potassium channel. Lipid effects. In Ion Channel Reconstitution. Plenum Press, New York. 469–482.
- Boldt, M., G. Burckhardt, and B.C. Burckhardt. 2003. NH4 + conductance in Xenopus laevis oocytes. III. Effect of NH3. Pflugers Arch. 446:652–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous