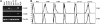Flk2+ myeloid progenitors are the main source of Langerhans cells - PubMed (original) (raw)
Flk2+ myeloid progenitors are the main source of Langerhans cells
Ines Mende et al. Blood. 2006.
Abstract
Langerhans cells (LCs) are antigen-presenting cells (APCs) residing in the epidermis that play a major role in skin immunity. Our earlier studies showed that when skin is inflamed LCs are replaced by bone marrow-derived progenitor cells, while during steady-state conditions LCs are able to self-renew in the skin. Identification of the LC progenitors in bone marrow would represent a critical step toward identifying the factors that regulate LC generation as well as their trafficking to the skin. To determine LC lineage origin, we reconstituted lethally irradiated CD45.2 mice with rigorously purified lymphoid and myeloid progenitors from CD45.1 congenic mice. Twenty-four hours later, we exposed the mice to UV light to deplete resident LCs and induce their replacement by progenitors. Reconstitution with common myeloid progenitors (CMPs), common lymphoid progenitors (CLPs), granulocyte-macrophage progenitors (GMPs), or early thymic progenitors led to LC generation within 2 to 3 weeks. CMPs were at least 20 times more efficient at generating LCs than CLPs. LCs from both lineages were derived almost entirely from fetal liver kinase-2+ (Flk-2+) progenitors, displayed typical dendritic-cell (DC) morphology, and showed long-term persistence in the skin. These results indicate that LCs are derived mainly from myeloid progenitors and are dependent on Flt3-ligand for their development.
Figures
Figure 1.
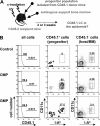
LCs develop from myeloid progenitors after UV light-induced skin inflammation. (A) Transplantation model. Lethally irradiated CD45.2 C57Bl/6 mice were injected intravenously with progenitor cells isolated from congenic CD45.1 mice plus 105 CD45.2 BM cells to ensure survival. Twenty-four hours after progenitor transplantation, mice received 20 minutes of UV light treatment to recruit progenitor cells to the skin. LC chimerism was analyzed by flow cytometry of epidermal cells. (B) Histogram plots show CD45.1 expression of epidermal cells isolated from mice 2 weeks after transplantation with 104 CMPs or 4 × 104 GMPs, or control mice. Control mice received transplants of autologous support BM cells only. Contour plots show CD11c/I-Ab expression profile of gated CD45.1+ and CD45.1- epidermal cells. Percentages of LCs derived from host- or donor-derived cells are indicated. Data are representative of at least 5 mice that underwent transplantation per progenitor population.
Figure 2.
LC reconstitution by lymphoid progenitor cells. (A) Histogram plots represent CD45.1 expression profile of epidermal-cell suspensions 2 and 3 weeks after adoptive transfer of either 104 CLPs or 5 × 104 pro-T1 or control cells (autologous BM only). Contour plots show corresponding I-Ab/CD11c expression profile of gated CD45.1+ and CD45.1- epidermal cells from mice that underwent transplantation. (B) Contour plot shows B220/TCRγδ profile of CLP-derived LCs (CD45.1+, I-Ab+, CD11c+ population) isolated from the epidermis of mice that received transplants of CLPs at 3 weeks after adoptive transfer. (C) Top contour plot shows CD45.1/CD45.2 expression profile of total spleen cells at 3 weeks after adoptive transfer of 104 CLPs. Bottom contour plot represents TCR/CD19 expression profile of gated CLP-derived (CD45.1+) spleen cells. (D) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells at 2 weeks (both myeloid and lymphoid progenitors) and 3 weeks (only CLPs, pro-T1, pro-T2) after adoptive transfer. ND indicates not determined. Data are representative of at least 5 transplantations per progenitor population with similar results.
Figure 3.
Progenitor-derived LCs persist in the skin. Epidermal cells were isolated from ears of chimeric mice 3 months after progenitor transfer. (A) Histograms in left panel show CD45.1 expression profile of total epidermal cells derived from CD45.2 mice reconstituted with either 104 CD45.1 CMPs, 4 × 104 GMPs, or 104 CLPs. Contour plots show CD11c/I-Ab expression profile of gated donor (CD45.1+) or host-derived (CD45.1-) epidermal cells. Percentages of LCs derived from transplanted cells (CD45.1+) or host cells (CD45.1-) are indicated. (B) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells at 3 months after adoptive transfer. Data are representative of at least 3 transplantations per progenitor population with similar results.
Figure 4.
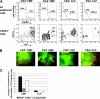
Morphology of CLP- and CMP-derived LCs. (A) Immunofluorescence staining with anti-CD45.1-PE and anti-I-Ab-FITC of epidermal sheets prepared from mice 3 months after transplantation and UV light exposure with either 104 CD45.1 CMPs or 104 CD45.1 CLPs. Epidermal sheets from CD45.2 mice that received transplants of only autologous BM cells served as controls. Scale bar: 10 μm. (B) Cytospin preparations of sorted CMP- or CLP-derived LCs. Sorted CD45.1+/I-Ab+/CD11c+/B220-/TCRγδ- epidermal cells derived from mice that received transplants of either CMPs or CLPs at 3 months after transfer were spun onto glass slides and stained with Wright solution. Scale bar: 10 μm. (C) RT-PCR analysis of Cd207 expression in sorted CD45.1+/I-Ab+/CD11c+/B220-/TCRγδ- epidermal cells. c-DNA was prepared at 4 weeks after transplantation from mice reconstituted with either 104 CD45.1 CMPs or 104 CD45.1 CLPs. cDNAs from LC numbers used per PCR reaction are given in brackets. Actb primers acted as controls. Data are based on at least 2 experiments with similar results.
Figure 5.
The potential to reconstitute LCs resides preferentially in the fraction of CLPs and CMPs expressing the Flt3 receptor. (A) Histograms in upper panel show CD45.1 expression profile of total epidermal cells derived from CD45.2 mice reconstituted with 104 CD45.1 CMPs or CLPs either expressing or lacking Flk2. Contour plots show CD11c/I-Ab expression profile of gated donor (CD45.1+) epidermal cells derived from the respective progenitor populations. Epidermal cells were analyzed for the presence of progenitor-derived LCs at 2 weeks (CMPs) or 3 weeks (CLPs). Percentages of LCs derived from transplanted cells (CD45.1+) or host cells (CD45.1-) are indicated. (B) Epidermal sheets from mice were prepared from mice that received transplants of 104 CD45.1+ CMPs (Flk2+ or Flk2-) at 2 weeks after transplantation and from mice reconstituted with either 104 CD45.1 Flk2+ CLPs or CD45.1 Flk2- CLPs at 3 weeks after transplantation. Shown are the overlays of photomicrographs obtained after immunofluorescence staining of epidermal sheets with anti-CD45.1-PE (red) and anti-I-Ab-FITC (green). Scale bar: 100 μm. Data are representative of at least 3 transplantations for each progenitor population with similar results. (C) Chart shows average LC reconstitution (% of total LCs) obtained per 104 progenitor cells (Flk2+, Flk2-, or unseparated) at 2 weeks (CMP populations) and 3 weeks (CLP populations) after adoptive transfer. Data are representative of at least 3 transplantations per progenitor population with similar results.
Figure 6.
Chemokine receptor expression on progenitors does not correlate with LC generation potential. (A) RT-PCR analysis of CCR2 and CCR6 expression in purified progenitor populations and epidermal LCs. Actb primers acted as controls. Data are based on 2 experiments with similar results. (B) Histogram plots show CCR2 and CCR6 (bold lines) surface expression of progenitor populations. Dashed lines represent isotype control stainings. Data are representative of 2 experiments with similar results.
Similar articles
- Langerhans cells renew in the skin throughout life under steady-state conditions.
Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Merad M, et al. Nat Immunol. 2002 Dec;3(12):1135-41. doi: 10.1038/ni852. Epub 2002 Nov 4. Nat Immunol. 2002. PMID: 12415265 Free PMC article. - Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors.
Karsunky H, Merad M, Mende I, Manz MG, Engleman EG, Weissman IL. Karsunky H, et al. Exp Hematol. 2005 Feb;33(2):173-81. doi: 10.1016/j.exphem.2004.10.010. Exp Hematol. 2005. PMID: 15676211 - Dendritic cell potentials of early lymphoid and myeloid progenitors.
Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Manz MG, et al. Blood. 2001 Jun 1;97(11):3333-41. doi: 10.1182/blood.v97.11.3333. Blood. 2001. PMID: 11369621 - Uncovering the Mysteries of Langerhans Cells, Inflammatory Dendritic Epidermal Cells, and Monocyte-Derived Langerhans Cell-Like Cells in the Epidermis.
Otsuka M, Egawa G, Kabashima K. Otsuka M, et al. Front Immunol. 2018 Jul 30;9:1768. doi: 10.3389/fimmu.2018.01768. eCollection 2018. Front Immunol. 2018. PMID: 30105033 Free PMC article. Review. - Establishing and maintaining the Langerhans cell network.
Chopin M, Nutt SL. Chopin M, et al. Semin Cell Dev Biol. 2015 May;41:23-9. doi: 10.1016/j.semcdb.2014.02.001. Epub 2014 Feb 7. Semin Cell Dev Biol. 2015. PMID: 24513231 Review.
Cited by
- Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages.
Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Karsunky H, et al. Blood. 2008 Jun 15;111(12):5562-70. doi: 10.1182/blood-2007-11-126219. Epub 2008 Apr 18. Blood. 2008. PMID: 18424665 Free PMC article. - Monocyte-mediated defense against microbial pathogens.
Serbina NV, Jia T, Hohl TM, Pamer EG. Serbina NV, et al. Annu Rev Immunol. 2008;26:421-52. doi: 10.1146/annurev.immunol.26.021607.090326. Annu Rev Immunol. 2008. PMID: 18303997 Free PMC article. Review. - Dendritic cell homeostasis.
Merad M, Manz MG. Merad M, et al. Blood. 2009 Apr 9;113(15):3418-27. doi: 10.1182/blood-2008-12-180646. Epub 2009 Jan 27. Blood. 2009. PMID: 19176316 Free PMC article. Review. - The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues.
Devi KS, Anandasabapathy N. Devi KS, et al. Semin Immunopathol. 2017 Feb;39(2):137-152. doi: 10.1007/s00281-016-0602-0. Epub 2016 Nov 25. Semin Immunopathol. 2017. PMID: 27888331 Free PMC article. Review. - The role of Langerhans cells in epidermal homeostasis and pathogenesis of psoriasis.
Yan B, Liu N, Li J, Li J, Zhu W, Kuang Y, Chen X, Peng C. Yan B, et al. J Cell Mol Med. 2020 Oct;24(20):11646-11655. doi: 10.1111/jcmm.15834. Epub 2020 Sep 11. J Cell Mol Med. 2020. PMID: 32916775 Free PMC article. Review.
References
- Stingl G, Tamaki K, Katz SI. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980; 53: 149-174. - PubMed
- Nestle FO, Nickoloff BJ. Dermal dendritic cells are important members of the skin immune system. Adv Exp Med Biol. 1995;378: 111-116. - PubMed
- Morhenn VB. Langerhans cells may trigger the psoriatic disease process via production of nitric oxide. Immunol Today. 1997;18: 433-436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous