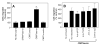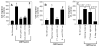Proteolysis of CCN1 by plasmin: functional implications - PubMed (original) (raw)
Proteolysis of CCN1 by plasmin: functional implications
Usha R Pendurthi et al. Cancer Res. 2005.
Abstract
Plasmin is shown to play a crucial role in many pathophysiologic processes primarily through its ability to degrade extracellular matrix (ECM) and/or mobilizing growth factors that are sequestered in the ECM. Cysteine-rich 61 (CCN1) is a matricellular protein of which expression is up-regulated in cancer and various vascular diseases. The present study was undertaken to investigate whether plasmin liberates CCN1 from the ECM and whether the released growth factor modulates endothelial cell migration. Treatment of breast carcinoma cells (MDA-MB-231) with plasmin released a truncated form of CCN1 (28 kDa) into the overlying medium. Experiments with recombinant CCN1 confirmed that plasmin effectively cleaves CCN1. Thrombin and other clotting/fibrinolytic proteases are ineffective in cleaving CCN1. Further studies revealed that the conditioned medium of plasmin-treated carcinoma cells supports endothelial cell migration and that antibodies specific to CCN1 blocked this enhancing effect. These data were the first to show that plasmin can liberate a pluripotent matrix signaling protein, CCN1, from the ECM. Because both CCN1 and the components of the plasmin generation system are present in tumor cells and a variety of other cells, the proteolysis of CCN1 by plasmin may play a role in many pathophysiologic processes, including tumor cell-mediated angiogenesis.
Figures
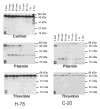
Figure 1
Plasmin cleavage of recombinant CCN1. Purified recombinant human CCN1-V5 fusion protein (10 μg/ml) was incubated with a control vehicle (A), plasmin (1 nM) (B and D) or thrombin (1 nM) (C and E). Aliquots removed at varying times (15 sec to 2 h) were subjected to SDS-PAGE, followed by immunoblot analysis with CCN1 antibodies (H-78 or C-20). Blots A, B and C were probed with H-78 antibody whereas blots D and E were probed with C-20 antibody.
Figure 2
Plasmin cleavage of cellular CCN1 and the release of CCN1 fragment into the overlying medium. MDA-MB-231 breast carcinoma cells, which constitutively express CCN1, were treated with plasmin or thrombin (10 nM) for varying times. Both the cell lysates and the overlying cell media were subjected to SDS-PAGE, followed by immunoblot analysis with H-78 or anti-ß-actin antibodies.
Figure 3
Effect of the conditioned media of MDA-MB-231 cells exposed to plasmin or thrombin on endothelial cell migration. (A) Conditioned media obtained from MDA-MB-231 cells treated with a control serum-free medium (CM/control), plasmin (10 nM) (CM/plasmin) or thrombin (10 nM) (CM/thrombin) were added to the bottom chamber of Transwell system and endothelial cells (50, 000) were placed in the upper chamber. The number of cells that migrated to the underside of the membrane in 6 h at 37°C was determined as described in methods (mean ± SE, n = 3 to 5). In parallel wells, plasmin (10 nM) was added to a well containing the serum-free medium with no cells and the medium was processed in the same way as the conditioned media derived from the cells treated with plasmin (SFM/plasmin). * denotes significantly differs from all other values ( p <0.005). (B) Neutralizing antibodies against bFGF (10 μg/ml), TGF-ß (10 μg/ml), IL-8 (50 μg/ml) or control IgG (50 μg/ml) were added to the conditioned media of plasmin-treated cells before it was placed in the bottom chamber of Transwell system. Cell migration was evaluated as in panel A. No statistical significant differences were found between the conditioned media (CM/plasmin) vs. the CM/plasmin treated with various antibodies (mean ± SE, n =4).
Figure 4
Plasmin-released CCN1 peptide fragment from MDA-MB-231 cells is responsible for endothelial cell migration. (A) Anti-CCN1 antibodies, either prepared in-house (lab) (40 μg/ml) or H-78 (25 μg/ml), or control IgG (40 μg/ml) were added to the conditioned media of plasmin-treated cells before it was added to the bottom wells of Transwell system. *, p <0.003 vs. control (no stimulant); **, p <0.01 vs. CM/plasmin or p <0.05 vs. CM/plasmin + control IgG; #, not significantly differs from CM/plasmin (p>0.6). (B) The bottom wells contained one of the following: the conditioned media of plasmin-treated cells (starting material), the conditioned media passed through anti-CCN1 antibody column (FT- anti-CCN1 col.) or the bound material eluted from the antibody column (E – anti-CCN1 col.). *, p <0.01 vs. control (no stimulant); **, p <0.01 vs. starting material; #, not significantly different from the starting material (p >0.4). (C) The bottom wells contained one of the following: the conditioned media of plasmin-treated cells (starting material), the conditioned media absorbed with heparin-agarose beads (FT- Hep. agarose) or the bound material eluted from the heparin agarose column (E – Hep. agarose). Endothelial cells (50, 000) were placed in the upper chamber and the number of cells that migrated to the underside of the membrane in 6 h at 37°C was determined as described in methods. The data shown are mean ± SE, n = 4 to 6 for A, n = 3 for B and C.
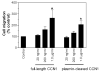
Figure 5
A comparison of full-length and plasmin-cleaved recombinant CCN1 in supporting endothelial cell migration. Recombinant CCN1 fusion protein (10 μg/ml) was digested with plasmin (1 nM) at 37°C for 15 min. Thereafter, plasmin was neutralized with PPACK (1 μM) for 30 min at room temperature and the samples were dialyzed overnight to remove free PPACK. Varying concentrations of full-length recombinant CCN1 or plasmin-cleaved CCN1 were added to the bottom chamber of Transwell system. Endothelial cells (50, 000) were placed in the upper chamber and the number of cells that migrated to the underside of the membrane in 6 h at 37°C was determined as described in methods. *, p <0.05 vs. control (no stimulant) (n=3).
Similar articles
- The matrix protein CCN1 (CYR61) promotes proliferation, migration and tube formation of endothelial progenitor cells.
Yu Y, Gao Y, Wang H, Huang L, Qin J, Guo R, Song M, Yu S, Chen J, Cui B, Gao P. Yu Y, et al. Exp Cell Res. 2008 Oct 15;314(17):3198-208. doi: 10.1016/j.yexcr.2008.08.001. Epub 2008 Aug 13. Exp Cell Res. 2008. PMID: 18755182 - The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration.
Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. Grote K, et al. Blood. 2007 Aug 1;110(3):877-85. doi: 10.1182/blood-2006-07-036202. Epub 2007 Apr 11. Blood. 2007. PMID: 17429007 Clinical Trial. - CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells.
Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. Si W, et al. Mol Cell Biol. 2006 Apr;26(8):2955-64. doi: 10.1128/MCB.26.8.2955-2964.2006. Mol Cell Biol. 2006. PMID: 16581771 Free PMC article. - Functional properties and intracellular signaling of CCN1/Cyr61.
Chen Y, Du XY. Chen Y, et al. J Cell Biochem. 2007 Apr 15;100(6):1337-45. doi: 10.1002/jcb.21194. J Cell Biochem. 2007. PMID: 17171641 Review. - All in the CCN family: essential matricellular signaling modulators emerge from the bunker.
Leask A, Abraham DJ. Leask A, et al. J Cell Sci. 2006 Dec 1;119(Pt 23):4803-10. doi: 10.1242/jcs.03270. J Cell Sci. 2006. PMID: 17130294 Review.
Cited by
- An overview of CCN4 (WISP1) role in human diseases.
Singh K, Oladipupo SS. Singh K, et al. J Transl Med. 2024 Jun 27;22(1):601. doi: 10.1186/s12967-024-05364-8. J Transl Med. 2024. PMID: 38937782 Free PMC article. Review. - Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61.
Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A, Chiocca EA, Kaur B. Kurozumi K, et al. Mol Ther. 2008 Aug;16(8):1382-91. doi: 10.1038/mt.2008.112. Epub 2008 Jun 10. Mol Ther. 2008. PMID: 18545226 Free PMC article. - Thrombin alters the synthesis and processing of CYR61/CCN1 in human corneal stromal fibroblasts and myofibroblasts through multiple distinct mechanisms.
Andreae EA, Warejcka DJ, Twining SS. Andreae EA, et al. Mol Vis. 2020 Jul 29;26:540-562. eCollection 2020. Mol Vis. 2020. PMID: 32818017 Free PMC article. - Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis.
Koon HW, Zhao D, Xu H, Bowe C, Moss A, Moyer MP, Pothoulakis C. Koon HW, et al. Am J Pathol. 2008 Aug;173(2):400-10. doi: 10.2353/ajpath.2008.080222. Epub 2008 Jul 3. Am J Pathol. 2008. PMID: 18599605 Free PMC article. - Plasminogen regulates mesenchymal stem cell-mediated tissue repair after ischemia through Cyr61 activation.
Duan H, He Z, Lin M, Wang Y, Yang F, Mitteer RA, Kim HJ, Yeo E, Han H, Qin L, Fan Y, Gong Y. Duan H, et al. JCI Insight. 2020 Aug 6;5(15):e131376. doi: 10.1172/jci.insight.131376. JCI Insight. 2020. PMID: 32759492 Free PMC article.
References
- Pepper MS. Role of the matrix metalloproteinases and plasminogen activator-plasmin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–17. - PubMed
- Lijnen HR. Plasmin and matrix metalloproteinases. Thromb Haemost. 2001;86:324–33. - PubMed
- Ploplis VA, Castellino FJ. Gene targeting of components of the fibrinolytic system. Thromb Haemost. 2002;87:22–31. - PubMed
- Carmeliet P, Collen D. Development and disease in proteinase-deficient mice: role of the plasminogen matrix metalloproteinase and coagulation system. Thromb Res. 1998;91:255–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058869-04/HL/NHLBI NIH HHS/United States
- HL 65500/HL/NHLBI NIH HHS/United States
- R01 HL065500/HL/NHLBI NIH HHS/United States
- R01 HL058869-05A3/HL/NHLBI NIH HHS/United States
- R01 HL058869-03/HL/NHLBI NIH HHS/United States
- R01 HL058869/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous