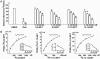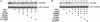The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase - PubMed (original) (raw)
The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase
Yi Liu et al. Proc Natl Acad Sci U S A. 2005.
Abstract
We previously reported that laminar flow activates peroxisome proliferator-activated receptor gamma (PPARgamma) in vascular endothelial cells in a ligand-dependent manner that involves phospholipase A2 and cytochrome P450 epoxygenases. In this study, we investigated whether epoxyeicosatrienoic acids (EETs), the catalytic products of cytochrome P450 epoxygenases, are PPARgamma ligands. Competition and direct binding assays revealed that EETs bind to the ligand-binding domain of PPARgamma with K(d) in the microM range. In the presence of adamantyl-ureido-dodecanoic acid (AUDA), a soluble epoxide hydrolase (sEH)-specific inhibitor, EETs increased PPARgamma transcription activity in endothelial cells and 3T3-L1 preadipocytes. Inclusion of AUDA in the perfusing media enhanced, but overexpression of sEH reduced, the laminar flow-induced PPARgamma activity. Furthermore, laminar flow augmented cellular levels of EETs but decreased sEH at the levels of mRNA, protein, and activity. Blocking PPARgamma by GW9662 abolished the EET/AUDA-mediated antiinflammatory effect, which indicates that PPARgamma is an effector of EETs.
Figures
Fig. 1.
EETs are PPARγ ligands. (A) One microgram of GST-PPARγ-LBD fusion protein was incubated with 100 nM [3H]rosiglitazone (Rosi) in a total volume of 50 μl in the presence of vehicle (DMSO), unlabeled rosiglitazone, or various EETs at the indicated concentrations. (B) For each data point, 1 μg of GST-PPARγ-LBD was incubated with various amounts of [3H]EETs in a total volume of 50 μl. The free and bound ligands in A and B were separated on a Sephadex G-25 column, and the amount of bound [3H]rosiglitazone or [3H]EETs was then determined by liquid scintillation counting. The results in A are presented as mean ± SD from three sets of experiments. *, P < 0.05 compared with DMSO controls. The plot of the binding curve in B represents three independent experiments, and Scatchard analysis was performed by replotting the data shown in Insets. _K_d was presented as mean ± SD of Scatchard analysis results from three independent experiments.
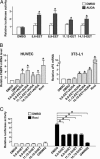
Fig. 2.
EETs, together with AUDA, activate the PPARγ-regulated transcription. (A) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc (0.25 μg), GAL-mPPARγ-LBD (0.25 μg), and CMV-_Renilla_-Luc (0.05 μg). The transfected cells were then incubated with EETs (1 μM) in the presence or absence of AUDA (1 μM), and the media were replaced every 2 h. After 8 h, cells were lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO controls. (B) Total RNAs were isolated from HUVECs or 3T3-L1 cells incubated with EETs (1 μM) in the presence of AUDA (1 μM) for 12 h. The levels of fatty acid-binding protein 4 mRNA in HUVECs or adipocyte P2 mRNA in 3T3-L1 cells were determined by quantitative RT-PCR in which β-actin was an internal control. The relative mRNA level is defined as the levels in cells treated with various EETs in reference to that of DMSO set as 1. (C) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc, GAL-mPPARγ-LBD, and CMV-_Renilla_-Luc. The transfected cells were then incubated with DHETs or GW9662 (5 μM) in the presence or absence of rosiglitazone (1 μM) for 8 h and lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO controls as 1. *, P < 0.05.
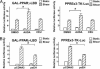
Fig. 3.
Inhibition of sEH in ECs enhances the response of PPARγ to laminar flow. (A) BAECs transfected with GAL-mPPARγ-LBD and MH100×4-Luc or PPRE×3-TK-Luc were kept as static controls or subjected to laminar flow for 8 h in the presence or absence of AUDA (1 μM), an sEH-specific inhibitor, or 1-cyclohexyl-3-ethyl urea (CEU, 1 μM), an AUDA analogue that does not inhibit sEH. (B) BAECs on glass slides were cotransfected with pCDNA3 (0.3 μg) or an expression plasmid encoding sEH (psEH, 0.3 μg) with GAL-mPPARγ-LBD (0.3 μg) and MH100×4-Luc (0.3 μg) or PPRE×3-TK-Luc (0.6 μg). The transfected cells were then kept as static controls or subjected to laminar flow for 8 h. Cells in both A and B were then lysed for luciferase activity assays. The bars represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of DMSO-treated static cells in A or pCDNA3-transfected cells under static conditions in B. *, P < 0.05.
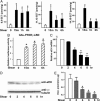
Fig. 4.
Laminar flow increases EETs and down-regulates sEH in ECs. BAECs in A, D, and E and HUVECs in C were kept as static controls or subjected to a laminar flow at 12 dyne/cm2 for the indicated times. (A) Total lipids were extracted from static BAECs or those exposed to laminar flow in the absence of FBS. EETs were quantified by LC/MS/MS. The amounts of EETs were normalized to the mass of the cell pellets (in grams). (B) BAECs in 12-well plates were cotransfected with MH100×4-TK-Luc, GAL-mPPARγ-LBD, and CMV-_Renilla_-Luc. The transfected cells were then incubated with the condition media collected from the flow experiments in A for 8 h and lysed for luciferase activity assays. The results represent the relative luciferase activity defined as the normalized luciferase activity of various experiments in reference to that of static medium controls as 1. (C) Total RNA was isolated from HUVECs, and the level of sEH mRNA was determined by quantitative RT-PCR with β-actin used as an internal control. (D) BAECs were lysed for immunoblotting with the use of polyclonal anti-sEH Ab. (E) Cytosolic supernatants obtained from BAECs were incubated with _trans_-[3H]stilbene oxide for sEH activity assays. *, P < 0.05, compared with static controls.
Fig. 5.
PPARγ mediates the antiinflammatory effect of EETs. BAECs were pretreated with AUDA (1 μM) for 2 h and then incubated with various EETs (1 μM) for 8 h. (B) Cells were cultured as in A, except that GW9662 (1 μM) was included in the indicated experiments during the 8-h incubation. All cells were then stimulated with TNF-α (10 ng/ml) for 30 min. The collected cell lysates were immunoblotted with anti-IκBα antibody. α-Tubulin was used as a loading control.
Similar articles
- Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells.
Liu Y, Zhu Y, Rannou F, Lee TS, Formentin K, Zeng L, Yuan X, Wang N, Chien S, Forman BM, Shyy JY. Liu Y, et al. Circulation. 2004 Aug 31;110(9):1128-33. doi: 10.1161/01.CIR.0000139850.08365.EC. Epub 2004 Aug 16. Circulation. 2004. PMID: 15313948 - Adenosine A2A receptor modulates vascular response in soluble epoxide hydrolase-null mice through CYP-epoxygenases and PPARγ.
Nayeem MA, Pradhan I, Mustafa SJ, Morisseau C, Falck JR, Zeldin DC. Nayeem MA, et al. Am J Physiol Regul Integr Comp Physiol. 2013 Jan 1;304(1):R23-32. doi: 10.1152/ajpregu.00213.2012. Epub 2012 Nov 14. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23152114 Free PMC article. - 17,18-epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase.
Morin C, Sirois M, Echavé V, Albadine R, Rousseau E. Morin C, et al. Am J Respir Cell Mol Biol. 2010 Nov;43(5):564-75. doi: 10.1165/rcmb.2009-0155OC. Epub 2009 Dec 11. Am J Respir Cell Mol Biol. 2010. PMID: 20008283 - [Soluble epoxide hydrolase and lipid metabolism].
Ma XX, Liu Y, Zhu Y. Ma XX, et al. Sheng Li Ke Xue Jin Zhan. 2010 Aug;41(4):267-71. Sheng Li Ke Xue Jin Zhan. 2010. PMID: 21416942 Review. Chinese. - Epoxyeicosatrienoic acid pathway in human health and diseases.
Bellien J, Joannides R. Bellien J, et al. J Cardiovasc Pharmacol. 2013 Mar;61(3):188-96. doi: 10.1097/FJC.0b013e318273b007. J Cardiovasc Pharmacol. 2013. PMID: 23011468 Review.
Cited by
- Dysregulation of the Arachidonic Acid Pathway in Cystic Fibrosis: Implications for Chronic Inflammation and Disease Progression.
D'Orazio S, Mattoscio D. D'Orazio S, et al. Pharmaceuticals (Basel). 2024 Sep 9;17(9):1185. doi: 10.3390/ph17091185. Pharmaceuticals (Basel). 2024. PMID: 39338347 Free PMC article. Review. - Design and Synthesis of Dual-Targeting Inhibitors of sEH and HDAC6 for the Treatment of Neuropathic Pain and Lipopolysaccharide-Induced Mortality.
Chen Y, Sun J, Tong H, Wang J, Cao R, Xu H, Chen L, Morisseau C, Zhang M, Shi Y, Han C, Zhuang J, Jing Y, Liu Z, Hammock BD, Chen G. Chen Y, et al. J Med Chem. 2024 Feb 8;67(3):2095-2117. doi: 10.1021/acs.jmedchem.3c02006. Epub 2024 Jan 18. J Med Chem. 2024. PMID: 38236416 - 1,3-Dichloroadamantyl-Containing Ureas as Potential Triple Inhibitors of Soluble Epoxide Hydrolase, p38 MAPK and c-Raf.
Gladkikh BP, Danilov DV, D'yachenko VS, Butov GM. Gladkikh BP, et al. Int J Mol Sci. 2023 Dec 26;25(1):338. doi: 10.3390/ijms25010338. Int J Mol Sci. 2023. PMID: 38203510 Free PMC article. - Crocin Ameliorates Diabetic Nephropathy through Regulating Metabolism, CYP4A11/PPARγ, and TGF-β/Smad Pathways in Mice.
Chen W, Su J, Liu Y, Gao T, Ji X, Li H, Li H, Wang Y, Zhang H, Lv S. Chen W, et al. Curr Drug Metab. 2023;24(10):709-722. doi: 10.2174/0113892002257928231031113337. Curr Drug Metab. 2023. PMID: 37936469 Free PMC article. - Hepatic Transcriptome and Its Regulation Following Soluble Epoxide Hydrolase Inhibition in Alcohol-Associated Liver Disease.
Warner JB, Hardesty JE, Song YL, Floyd AT, Deng Z, Jebet A, He L, Zhang X, McClain CJ, Hammock BD, Warner DR, Kirpich IA. Warner JB, et al. Am J Pathol. 2024 Jan;194(1):71-84. doi: 10.1016/j.ajpath.2023.09.016. Epub 2023 Nov 3. Am J Pathol. 2024. PMID: 37925018 Free PMC article.
References
- Wissler, R. W. (1995) Am. J. Med. Sci. 310, Suppl. 1, 29-36. - PubMed
- Tsao, P. S., Buitrago, R., Chan, J. R. & Cooke, J. P. (1996) Circulation 94, 1682-1689. - PubMed
- Plutzky, J. (2001) Curr. Opin. Lipidol. 12, 511-518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL77448/HL/NHLBI NIH HHS/United States
- F32 ES005707/ES/NIEHS NIH HHS/United States
- ES02710/ES/NIEHS NIH HHS/United States
- R01 HL072845/HL/NHLBI NIH HHS/United States
- R01 ES002710/ES/NIEHS NIH HHS/United States
- P30 ES005707/ES/NIEHS NIH HHS/United States
- P42 ES004699/ES/NIEHS NIH HHS/United States
- ES05707/ES/NIEHS NIH HHS/United States
- R01 HL077448/HL/NHLBI NIH HHS/United States
- ES04699/ES/NIEHS NIH HHS/United States
- HL72845/HL/NHLBI NIH HHS/United States
- R37 ES002710/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources