Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia - PubMed (original) (raw)
Comparative Study
Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia
Carsten Culmsee et al. J Neurosci. 2005.
Erratum in
- J Neurosci. 2005 Nov 16;25(46):table of contents. Pellechia, Maurizio [corrected to Pellecchia, Maurizio]
Abstract
Delayed neuronal cell death occurring hours after reperfusion is a hallmark of ischemic stroke and a primary target for neuroprotective strategies. In the present study, we investigated whether apoptosis-inducing factor (AIF), a caspase-independent proapoptotic protein, is responsible for neuronal cell death after glutamate toxicity and oxygen-glucose deprivation (OGD) in vitro and after experimental stroke in vivo. AIF translocated to the nucleus in which it colocalized with DNA fragmentation and nuclear apoptotic morphology after exposure to glutamate or OGD in cultured neurons or after transient middle cerebral artery occlusion (MCAo) in mice. Small inhibitory RNA-mediated downregulation of AIF reduced glutamate- and OGD-induced neuronal apoptosis by 37 and 60%, respectively (p < 0.01). Moreover, Harlequin mutant mice, which express AIF at low levels (approximately 20% of wild-type mice), displayed smaller infarct volumes (-43%; p < 0.03) and showed dramatically reduced cell death in the ischemic penumbra after 45 min of MCAo compared with wild-type littermates. Inhibition of poly(ADP-ribose) polymerase and Bid reduced nuclear AIF translocation. These results provide the first evidence for a causal role of AIF in ischemic neuronal cell death. Therefore, caspase-independent cell death signaling may provide a promising novel target for therapeutic interventions in cerebrovascular diseases.
Figures
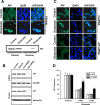
Figure 1.
AIF-siRNA knock-down attenuates glutamate-induced neuronal cell death in hippocampal neurons. A, Top row, Confocal laser scanning microscope images of AIF immunoreactivity (green) under control conditions and after 18 h exposure to glutamate (2 m
m
) in immortalized hippocampal neurons (HT22 cells). Costaining with DAPI (dark blue) allowed the identification of nuclear translocation of AIF (AIF/DAPI, light blue). Bottom row, Western blot analysis of AIF protein in the crude cytosolic and in the nuclear fractions before and after exposure to 2 m
m
glutamate. B, RT-PCR analysis of AIF RNA (top 2 rows) and Western blot analysis of AIF protein levels (bottom 2 row) in HT22 cells pretreated with 20 n
m
AIF siRNA for 48 h. Controls were treated with mutant siRNA. RT-PCR with primers specific for GAPDH and anti-β-actin antibodies were used as controls for equal mRNA and protein amounts, respectively. HT22 cells were pretreated with vehicle (Lipofectamine), nonfunctional mutant siRNA (mut-siRNA), or AIF-siRNA for 48 h. C, Confocal laser scanning microscope images of AIF immunoreactivity (green) and nuclear DAPI staining (dark blue). Downregulation of AIF by siRNA (see above) resulted in inhibition of nuclear AIF translocation after 18 h of exposure to glutamate (2 m
m
). D, Cell viability was determined by the MTT assay, 20 n
m
mutants iRNA, or AIF-siRNA for 48 h before 18 h 1-2 m
m
glutamate exposure. Cell viability in glutamate-exposed cultures pretreated with AIF-siRNA was significantly enhanced compared with controls (n = 8; *p < 0.001).
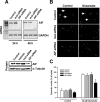
Figure 2.
Reduced glutamate-induced apoptosis in primary neurons pretreated with AIF-siRNA. A, AIF mRNA and protein were significantly downregulated, as demonstrated by RT-PCR (top row) and Western blot analysis (bottom row), respectively, in primary hippocampal neurons pretreated with AIF siRNA for 24 or 48 h. RT-PCR for GAPDH (bottom) and Western blotting for α-tubulin were performed as controls. B, Fluorescence microscope images of DAPI-stained embryonic hippocampal neurons were obtained after 48 h of exposure to 20 μ
m
glutamate in Locke's medium. Only cultures pretreated with 20 n
m
AIF-siRNA contained ∼50% healthy nuclei, whereas all other glutamate-treated groups showed >85% pyknotic and/or fragmented nuclei, indicating apoptotic damage (Glutamate, right column). The respective control cultures with Locke's medium contained only very few apoptotic nuclei (Control, left column). C, Primary hippocampal neurons were left untreated (Controls) or were pretreated with vehicle (Lipofectamine), 20 n
m
nonfunctional mutant siRNA (mut-siRNA), or 20 n
m
AIF-siRNA for 48 h. On DIV9, cells were exposed to 20 μ
m
glutamate in Locke's medium for 48 h. Thereafter, neurons were fixed with paraformaldehyde, and apoptotic nuclei were quantified after staining with DAPI. Pretreatment with AIF-siRNA significantly reduced apoptotic cell death compared with the other cultures exposed to glutamate (n = 4; *p < 0.01 vs all other glutamate-treated groups).
Figure 3.
Reduced ischemic cell death after oxygen/glucose deprivation in primary neurons pretreated with AIF-siRNA. A, In control neurons (top row), AIF (green) is located outside the DAPI-stained nucleus (dark blue). Oxygen-glucose deprivation (8 h) caused translocation of AIF (green) to the nucleus (dark blue) 4 h after reoxygenation. B, Number of damaged neurons and neurons displaying nuclear AIF 4 and 8 h after reoxygenation after 4 h of oxygen-glucose deprivation. AIF translocates to the nucleus before signs of morphological neuronal damage [as determined by nuclear morphology after DAPI/Hoechst (Hoe) staining or propidium iodide/calcein staining] become evident. C, Primary hippocampal neurons were left untreated (Controls) or were pretreated with vehicle (Lipofectamine), 20 n
m
nonfunctional mutant siRNA (mut-siRNA), or 20 n
m
AIF-siRNA for 48 h. On DIV9, cells were exposed to 4 h of oxygen-glucose deprivation. Thereafter, neurons were fixed with paraformaldehyde, and apoptotic nuclei were quantified after staining with DAPI. Neurons pretreated with AIF-siRNA showed less pyknosis after OGD compared with nontreated neurons. D, Quantification of experiments described in C. Pretreatment with AIF-siRNA significantly reduced apoptotic cell death compared with control cultures (n = 4; *p < 0.01 vs all other glutamate-treated groups).
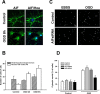
Figure 4.
PARP1 and Bid inhibition reduced nuclear AIF translocation after oxygen-glucose deprivation in primary neurons. A, In control neurons (top row), AIF (green) is located outside the DAPI-stained nucleus (dark blue). Oxygen-glucose deprivation (8 h) caused translocation of AIF (green) to the nucleus (dark blue; middle row), a finding that was almost absent in cultures pretreated with 1 μ
m
of the PARP1 inhibitor PJ-34 (bottom row). B, Number of DAPI-stained neurons displaying pyknotic nuclei before and after 4 h of OGD. Neurons pretreated with 1 μ
m
PARP1 inhibitor PJ-34 were significantly protected from hypoxic-hypoglycemic cell death (*p < 0.01 vs untreated cells). C, Similar to previous experiments, control neurons (top row) displayed mitochondrial distribution of AIF (green) that translocated to the nucleus after OGD (middle row). Neurons pretreated with 2 μ
m
Bid inhibitor Bl6c9 showed no nuclear AIF staining after OGD (bottom row). Scale bar, 20 μm. D, Counting of DAPI-stained neurons displaying pyknotic nuclei before and after 4 h of OGD revealed that 2 μ
m
Bid inhibitor Bl6c9 significantly protected neurons from hypoxic-hypoglycemic cell death (*p < 0.01 vs untreated neurons subjected to OGD). Control neurons were treated with 2 μ
m
(data not shown) and 10 μ
m
BI6c9 to evaluate compound toxicity.
Figure 5.
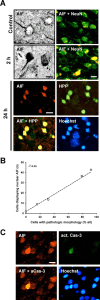
AIF translocates to the nucleus and is associated with DNA damage after focal cerebral ischemia. A, Photomicrographs of C57BL/6 mouse brain sections showing cytoplasmic AIF immunostaining in normal neurons, as demonstrated by morphology and by colocalization with the neuronal marker NeuN (Control), and extensive nuclear translocation after transient MCAo (2 h). At 24 h after ischemia, nuclear AIF immunoreactivity (red) was colocalized with the DNA damage marker HPP (green), both of which appeared only in cells displaying condensed, pyknotic nuclei, as judged by Hoechst chromatin staining (blue). Scale bar, 10 μm. B, Cells with intense AIF immunoreactivity in the nucleus were counted in the striatum and in cortex 1-24 h after transient MCAo and plotted against the number of cells with a morphology indicating cell death in adjacent, Nissl-stained sections. The close correlation between the two parameters (_r_2 = 0.99; p < 0.001) indicates that nuclear translocation of AIF is closely associated to post-ischemic cell death in vivo (n = 4 per group). C, During the first hours of recovery after MCAo, there are multiple cells displaying nuclear AIF (red) but only scattered cells positive for active caspase-3 (green), indicating that AIF translocation either precedes activation of caspase-3 or displays an alternative signaling pathway of cell death in neurons.
Figure 6.
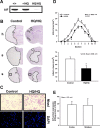
Significant reduction of ischemic brain damage in AIF low-expressing mice. A, Western blot analysis shows markedly reduced AIF protein levels in the brains of HQ/HQ mice compared with homozygote wild-type (+/+) and heterozygote (+/HQ) littermates. B, Nissl-stained brain sections of hippocampal (h) and striatal (s) brain regions obtained 24 h after reperfusion demonstrate significant reduction of infarcted brain tissue in Harlequin mice compared with littermate controls. The squares indicate the areas shown at higher magnification in C. C, High-magnification photomicrographs of Nissl-stained sections from cortical areas indicated by squares in B show shrunken cells with pyknotic nuclei in the infarct area of wild-type littermates (Control), whereas the cells in the corresponding area in HQ mice show normal morphology (top row). Fluorescent photomicrographs of respective cortical areas in adjacent sections stained with DAPI show that all damaged neurons in the infarct area of wild-type animals have pyknotic nuclei, whereas almost all nuclei in the corresponding area in HQ mice appear normal (bottom row). D, Infarct areas were evaluated histomorphometrically on 11 consecutive Nissl-stained brain sections (500 μm apart) throughout the infarct (top row). Note that, in each section, the mean infarct area was reduced in HQ mice compared with controls. The infarct volume was calculated on the basis of the histomorphometric data from the individual sections, showing a 43% reduction of the mean infarct volume in HQ mice compared with wild-type littermates (bottom row; *p < 0.03; n = 5 per group). E, HQ mice displayed equal expression of AIF in cerebral cortex and striatum as shown by Western blot analysis (n = 4).
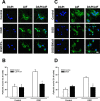
Figure 7.
PARP1 inhibition reduces AIF translocation after cerebral ischemia. A, Immunohistochemical staining of AIF (black) in the cerebral cortex of control animals and of mice receiving 10 mg/kg of the selective PARP inhibitor PJ-34 intraperitoneally 15 min before MCAo. PARP inhibition reduces the number of neurons displaying nuclear AIF translocation. B, Number of neurons in cerebral cortex and striatum displaying nuclear AIF or active caspase-3 staining (striatum only) in control animals (white bars) and in animals receiving a PARP inhibitor (black bars; see above). PARP inhibition reduced the number of neurons showing nuclear AIF by 40-45% (n = 5; p < 0.01) but did not effect caspase-3 activation.
Similar articles
- Endonuclease G does not play an obligatory role in poly(ADP-ribose) polymerase-dependent cell death after transient focal cerebral ischemia.
Xu Z, Zhang J, David KK, Yang ZJ, Li X, Dawson TM, Dawson VL, Koehler RC. Xu Z, et al. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R215-21. doi: 10.1152/ajpregu.00747.2009. Epub 2010 Apr 28. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20427721 Free PMC article. - Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats.
Li WH, Yang YL, Cheng X, Liu M, Zhang SS, Wang YH, Du GH. Li WH, et al. Apoptosis. 2020 Jun;25(5-6):354-369. doi: 10.1007/s10495-020-01600-w. Apoptosis. 2020. PMID: 32338336 - Contributions of poly(ADP-ribose) polymerase-1 and -2 to nuclear translocation of apoptosis-inducing factor and injury from focal cerebral ischemia.
Li X, Klaus JA, Zhang J, Xu Z, Kibler KK, Andrabi SA, Rao K, Yang ZJ, Dawson TM, Dawson VL, Koehler RC. Li X, et al. J Neurochem. 2010 May;113(4):1012-22. doi: 10.1111/j.1471-4159.2010.06667.x. Epub 2010 Mar 4. J Neurochem. 2010. PMID: 20236222 Free PMC article. - Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.
Ferrer I, Planas AM. Ferrer I, et al. J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review. - Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion.
van Wijk SJ, Hageman GJ. van Wijk SJ, et al. Free Radic Biol Med. 2005 Jul 1;39(1):81-90. doi: 10.1016/j.freeradbiomed.2005.03.021. Epub 2005 Apr 8. Free Radic Biol Med. 2005. PMID: 15925280 Review.
Cited by
- Therapeutic applications of PARP inhibitors: anticancer therapy and beyond.
Curtin NJ, Szabo C. Curtin NJ, et al. Mol Aspects Med. 2013 Dec;34(6):1217-56. doi: 10.1016/j.mam.2013.01.006. Epub 2013 Jan 29. Mol Aspects Med. 2013. PMID: 23370117 Free PMC article. Review. - Lepidium sativum as candidate against excitotoxicity in retinal ganglion cells.
Al-Dbass A, Amina M, Al Musayeib NM, El-Anssary AA, Bhat RS, Fahmy R, Alhamdan MM, El-Ansary A. Al-Dbass A, et al. Transl Neurosci. 2021 Jun 4;12(1):247-259. doi: 10.1515/tnsci-2020-0174. eCollection 2021 Jan 1. Transl Neurosci. 2021. PMID: 34141454 Free PMC article. - Endonuclease G does not play an obligatory role in poly(ADP-ribose) polymerase-dependent cell death after transient focal cerebral ischemia.
Xu Z, Zhang J, David KK, Yang ZJ, Li X, Dawson TM, Dawson VL, Koehler RC. Xu Z, et al. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R215-21. doi: 10.1152/ajpregu.00747.2009. Epub 2010 Apr 28. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20427721 Free PMC article. - Nuclear poly(ADP-ribose) activity is a therapeutic target in amyotrophic lateral sclerosis.
McGurk L, Mojsilovic-Petrovic J, Van Deerlin VM, Shorter J, Kalb RG, Lee VM, Trojanowski JQ, Lee EB, Bonini NM. McGurk L, et al. Acta Neuropathol Commun. 2018 Aug 29;6(1):84. doi: 10.1186/s40478-018-0586-1. Acta Neuropathol Commun. 2018. PMID: 30157956 Free PMC article. - Pathways to ischemic neuronal cell death: are sex differences relevant?
Lang JT, McCullough LD. Lang JT, et al. J Transl Med. 2008 Jun 23;6:33. doi: 10.1186/1479-5876-6-33. J Transl Med. 2008. PMID: 18573200 Free PMC article. Review.
References
- Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, Endres M (2001) Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med 7: 255-260. - PubMed
- Becattini B, Sareth S, Zhai D, Crowell KJ, Leone M, Reed JC, Pellecchia M (2004) Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol 11: 1107-1117. - PubMed
- Cande C, Vahsen N, Garrido C, Kroemer G (2004) Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ 11: 591-595. - PubMed
- Cao G, Clark RS, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J (2003) Translocation of apoptosis-inducing factor in vulnerable neurons after transient cerebral ischemia and in neuronal cultures after oxygen-glucose deprivation. J Cereb Blood Flow Metab 23: 1137-1150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources