Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells - PubMed (original) (raw)
Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells
David A Hess et al. Blood. 2006.
Abstract
The development of novel cell-based therapies requires understanding of distinct human hematopoietic stem and progenitor cell populations. We recently isolated reconstituting hematopoietic stem cells (HSCs) by lineage depletion and purification based on high aldehyde dehydrogenase activity (ALDH(hi)Lin- cells). Here, we further dissected the ALDH(hi)-Lin- population by selection for CD133, a surface molecule expressed on progenitors from hematopoietic, endothelial, and neural lineages. ALDH(hi)CD133+Lin- cells were primarily CD34+, but also included CD34-CD38-CD133+ cells, a phenotype previously associated with repopulating function. Both ALDH(hi)CD133-Lin- and ALDH(hi)CD133+Lin- cells demonstrated distinct clonogenic progenitor function in vitro, whereas only the ALDH(hi)CD133+Lin- population seeded the murine bone marrow 48 hours after transplantation. Significant human cell repopulation was observed only in NOD/SCID and NOD/SCID beta2M-null mice that received transplants of ALDH(hi)CD133+Lin- cells. Limiting dilution analysis demonstrated a 10-fold increase in the frequency of NOD/SCID repopulating cells compared with CD133+Lin- cells, suggesting that high ALDH activity further purified cells with repopulating function. Transplanted ALDH(hi)CD133+Lin- cells also maintained primitive hematopoietic phenotypes (CD34+CD38-) and demonstrated enhanced repopulating function in recipients of serial, secondary transplants. Cell selection based on ALDH activity and CD133 expression provides a novel purification of HSCs with long-term repopulating function and may be considered an alternative to CD34 cell selection for stem cell therapies.
Figures
Figure 1.
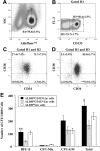
Isolation and in vitro progenitor activity of purified ALDHhiCD133-Lin- and ALDHhiCD133+Lin- cell populations. (A) Lin- cells incubated with Aldefluor substrate were used to select ALDHhi cells (R1, 55.8% ± 3.5%). (B) Staining for CD133 expression revealed the ALDHhiCD133-Lin- (R2 = 33.7% ± 1.7%) and ALDHhiCD133+Lin- (R3 = 50.4% ± 2.5%) purified populations. (C-D) Isolated ALDHhiCD133-Lin- and ALDHhiCD133+Lin- sorted cells were analyzed for CD34 and CD38 expression. Purified ALDHhiCD133+Lin- cells were enriched for repopulating CD34+CD38- cells (**P < .01) and included primitive CD34-CD38- cells. Data represent the mean ± SEM for cells isolated from 10 UCB samples. (E) Purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells were cultured in methylcellulose media and erythrocyte, mixed, and granulocyte/macrophage colonies (BFU-E, Mix, CFU-GM) were enumerated after 14 to 17 days of in vitro culture. Data represent the number of individual colonies produced per 1000 cells plated from each population. Data are expressed as mean ± SEM for cells isolated from 4 to 6 UCB Lin- samples (*P < .05; **P < .01).
Figure 2.
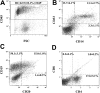
BM homing of purified ALDHhiCD133+Lin- or ALDHhiCD133-Lin- cells 48 hours after transplantation. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of 2 × 105 purified (A-C) ALDHhiCD133-Lin- or (D-F) ALDHhiCD133+Lin- cells. At 48 hours after transplantation, human hematopoietic cells were detected in the murine BM by coexpression of CD45 (R1) and HLA A, B, and C (R2).
Figure 3.
Detection of human cell repopulation in mice that received transplants of purified ALDHhiCD133-Lin-, ALDHhiCD133+Lin-, or ALDHhiLin- cells. Representative flow cytometric analysis of NOD/SCID β2M-null mice that received transplants of (A-C) 2 × 105 ALDHhiCD133-Lin-, (D-F) 104 ALDHhiCD133+Lin-, or (G-I) 104 ALDHhiLin- cells. At 7 to 8 weeks after transplantation, human hematopoietic cells in the mouse BM were detected by coexpression of CD45 (R1) with CD38 (R2). Cell suspensions from murine spleen and peripheral blood were analyzed in an identical fashion. Injection of more than 2 × 105 ALDHhiCD133-Lin- cells was required to observe human cell engraftment in the BM of NOD/SCID β2M-null mice (n = 3). Mice that received transplants of 104 ALDHhiCD133+Lin- cells showed enhanced engraftment with human cells (18.2% ± 10.9%, n = 6) in the murine BM, compared with mice that received transplants of 104 ALDHhiLin- cells not selected for CD133 expression (5.2% ± 1.7%, n = 5).
Figure 4.
Summary of human cell repopulation in mice that received transplants of ALDHhiCD133-Lin- or ALDHhiCD133+Lin- cells. A summary of the level of human engraftment in the BM (A-B), spleen (C-D), and peripheral blood (E-F) of NOD/SCID β2M-null mice (A,C,E; n = 33) or NOD/SCID mice (B,D,F; n = 41) that received transplants of purified 5 × 104 to 4 × 105 ALDHhiCD133-Lin- (▪) or 2 × 102 to 105 purified ALDHhiCD133+Lin- (•) cells. Horizontal bars represent the average level of human engraftment (mean ± SEM) at 104 or 105 injected cells. The frequency of BM repopulating cells by LDA was 1 SRC in 485 ALDHhiCD133+Lin- cells in the NOD/SCID β2M-null mouse or 1 SRC in 16 064 ALDHhiCD133+Lin- in the NOD/SCID mouse. Mice received transplants of the purified cells from 34 cord blood donors.
Figure 5.
Transplanted human ALDHhiCD133+Lin- cells differentiate into lymphoid and myeloid progeny in vivo. BM from highly engrafted mice that received transplants of 104 to 105 ALDHhiCD133+Lin- cells was stained with human-specific antibodies for mature hematopoietic lineage markers. (A) Human hematopoietic cells were selected by the expression of human CD45 (R2 = 62.5% ± 10.1%, n = 6) and analyzed for myeloid cell markers CD14 and CD33 (B), B-lymphocyte markers CD20 and CD19 (C), and T-lymphocyte markers CD4 and CD8 (D). Lymphoid and myeloid differentiation was observed after the transplantation of purified ALDHhiCD133+Lin- cells. T-lymphocyte production was not supported in the NOD/SCID β2M-null or NOD/SCID mouse.
Figure 6.
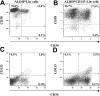
Transplanted human ALDHhiCD133+Lin- cells retain primitive hematopoietic phenotypes. BM from highly engrafted (24.9%-86.6% human CD45+) NOD/SCID β2M-null mice was analyzed for the maintenance of primitive cell surface phenotype 7 to 8 weeks after transplantation. Human cells were analyzed for the coexpression of CD34 with CD38 (A-B) or CD34 with CD133 (C-D).
Similar articles
- Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity.
Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA. Hess DA, et al. Blood. 2004 Sep 15;104(6):1648-55. doi: 10.1182/blood-2004-02-0448. Epub 2004 Jun 3. Blood. 2004. PMID: 15178579 - Coculture and transplant of purified CD34(+)Lin(-) and CD34(-)Lin(-) cells reveals functional interaction between repopulating hematopoietic stem cells.
Hess DA, Karanu FN, Levac K, Gallacher L, Bhatia M. Hess DA, et al. Leukemia. 2003 Aug;17(8):1613-25. doi: 10.1038/sj.leu.2403028. Leukemia. 2003. PMID: 12886251 - Dual SP/ALDH functionalities refine the human hematopoietic Lin-CD34+CD38- stem/progenitor cell compartment.
Pierre-Louis O, Clay D, Brunet de la Grange P, Blazsek I, Desterke C, Guerton B, Blondeau C, Malfuson JV, Prat M, Bennaceur-Griscelli A, Lataillade JJ, Le Bousse-Kerdilès MC. Pierre-Louis O, et al. Stem Cells. 2009 Oct;27(10):2552-62. doi: 10.1002/stem.186. Stem Cells. 2009. PMID: 19650038 - [Updated human hematopoietic stem cell findings: purification of human hematopoietic stem cells and elucidation of their hierarchy].
Sonoda Y. Sonoda Y. Rinsho Ketsueki. 2018;59(10):1861-1871. doi: 10.11406/rinketsu.59.1861. Rinsho Ketsueki. 2018. PMID: 30305486 Review. Japanese. - Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions.
Lapidot T. Lapidot T. Ann N Y Acad Sci. 2001 Jun;938:83-95. doi: 10.1111/j.1749-6632.2001.tb03577.x. Ann N Y Acad Sci. 2001. PMID: 11458529 Review.
Cited by
- CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells.
Arndt K, Grinenko T, Mende N, Reichert D, Portz M, Ripich T, Carmeliet P, Corbeil D, Waskow C. Arndt K, et al. Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5582-7. doi: 10.1073/pnas.1215438110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509298 Free PMC article. - CD133-targeted gene transfer into long-term repopulating hematopoietic stem cells.
Brendel C, Goebel B, Daniela A, Brugman M, Kneissl S, Schwäble J, Kaufmann KB, Müller-Kuller U, Kunkel H, Chen-Wichmann L, Abel T, Serve H, Bystrykh L, Buchholz CJ, Grez M. Brendel C, et al. Mol Ther. 2015 Jan;23(1):63-70. doi: 10.1038/mt.2014.173. Epub 2014 Sep 5. Mol Ther. 2015. PMID: 25189742 Free PMC article. - Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor.
Hess DA, Bonde J, Craft TP, Wirthlin L, Hohm S, Lahey R, Todt LM, Dipersio JF, Devine SM, Nolta JA. Hess DA, et al. Biol Blood Marrow Transplant. 2007 Apr;13(4):398-411. doi: 10.1016/j.bbmt.2006.12.445. Biol Blood Marrow Transplant. 2007. PMID: 17382247 Free PMC article. - Aldehyde dehydrogenase activity identifies a population of human skeletal muscle cells with high myogenic capacities.
Vauchez K, Marolleau JP, Schmid M, Khattar P, Chapel A, Catelain C, Lecourt S, Larghéro J, Fiszman M, Vilquin JT. Vauchez K, et al. Mol Ther. 2009 Nov;17(11):1948-58. doi: 10.1038/mt.2009.204. Epub 2009 Sep 8. Mol Ther. 2009. PMID: 19738599 Free PMC article. - Selection of tumorigenic melanoma cells using ALDH.
Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y, Leong SP, Smith JE, Ghadially R. Boonyaratanakornkit JB, et al. J Invest Dermatol. 2010 Dec;130(12):2799-808. doi: 10.1038/jid.2010.237. Epub 2010 Aug 26. J Invest Dermatol. 2010. PMID: 20739950 Free PMC article.
References
- Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis: III, a hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133: 157-165. - PubMed
- Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4: 1038-1045. - PubMed
- Dao MA, Arevalo J, Nolta JA. Reversibility of CD34 expression on human hematopoietic stem cells that retain the capacity for secondary reconstitution. Blood. 2003;101: 112-118. - PubMed
- Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95: 102-110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01 DK53041/DK/NIDDK NIH HHS/United States
- R01 DK061848-04/DK/NIDDK NIH HHS/United States
- R01 DK053041/DK/NIDDK NIH HHS/United States
- R01 DK061848/DK/NIDDK NIH HHS/United States
- P01 HL54850/HL/NHLBI NIH HHS/United States
- R01 DK61848/DK/NIDDK NIH HHS/United States
- R01 DK053041-08/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials