WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability - PubMed (original) (raw)
WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability
Kristopher T Kahle et al. Proc Natl Acad Sci U S A. 2005.
Abstract
The regulation of Cl(-) transport into and out of cells plays a critical role in the maintenance of intracellular volume and the excitability of GABA responsive neurons. The molecular determinants of these seemingly diverse processes are related ion cotransporters: Cl(-) influx is mediated by the Na-K-2Cl cotransporter NKCC1 and Cl(-) efflux via K-Cl cotransporters, KCC1 or KCC2. A Cl(-)/volume-sensitive kinase has been proposed to coordinately regulate these activities via altered phosphorylation of the transporters; phosphorylation activates NKCC1 while inhibiting KCCs, and dephosphorylation has the opposite effects. We show that WNK3, a member of the WNK family of serine-threonine kinases, colocalizes with NKCC1 and KCC1/2 in diverse Cl(-)-transporting epithelia and in neurons expressing ionotropic GABA(A) receptors in the hippocampus, cerebellum, cerebral cortex, and reticular activating system. By expression studies in Xenopus oocytes, we show that kinase-active WNK3 increases Cl(-) influx via NKCC1, and that it inhibits Cl(-) exit through KCC1 and KCC2; kinase-inactive WNK3 has the opposite effects. WNK3's effects are imparted via altered phosphorylation and surface expression of its downstream targets and bypass the normal requirement of altered tonicity for activation of these transporters. Together, these data indicate that WNK3 can modulate the level of intracellular Cl(-) via opposing actions on entry and exit pathways. They suggest that WNK3 is part of the Cl(-)/volume-sensing mechanism necessary for the maintenance of cell volume during osmotic stress and the dynamic modulation of GABA neurotransmission.
Figures
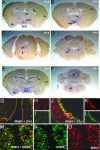
Fig. 1.
WNK3 expression in brain. (A-F) Antisense riboprobe to WNK3 was prepared, hybridized in situ to mouse brain sections, and visualized as described in Methods. Coronal sections at postnatal day 15 (P15; A-D) and P21 (E and F) are shown. (A) WNK3 expression is high in the hippocampus (Hp), thalamus (Th), layers of the cerebral (Cx), and entorhinal (En) cortices and in the supraoptic (SON) and suprachiasmatic nuclei (SCN). (B) Strong WNK3 expression is seen in hypothalamus (Hyp), Cx, and thalamus (Th). (C) WNK3 is expressed in the dorsal raphe nucleus (DR). (D) Intense WNK3 expression in the locus ceruleus (LC). (E) WNK3 expression is higher at P21, especially in Hp, Cx, and En. WNK3 expression in Hyp is also strong. (F) At P21, WNK3 is expressed in the Purkinje layer (arrow) of the cerebellum (Cb) and the medullary reticular formation (RF). (G-L) Expression in brain epithelia. Sections were stained with anti-WNK3 (red) and anti-zona-occludens-1 (green). WNK3 localizes to intercellular junctions in the epithelium (arrow) lining the third and lateral ventricles (Vn) of mouse brain. (Original magnification: _G_-I, ×200; _J_-L, ×630.) (M-O) Expression in brain parenchyma. Sections were stained with anti-WNK3 (red) and anti-GABAA receptor (green). A section of hippocampus is shown. WNK3 localizes to the cell bodies of neurons expressing the ionotropic GABAA receptor; similar results are seen in cerebellum and cortex. (Original magnification: ×350.)
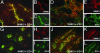
Fig. 2.
Localization of WNK3 in Cl--transporting extrarenal epithelia. Frozen mouse tissue sections were stained with anti-WNK3 (red) and anti-zona-occludens-1 (green) and analyzed with immunofluoresence light microscopy. Composite views (large) and staining by each antibody alone (small) are shown. (A-C) Pancreas. WNK3 localizes to intercellular junctions of large main exocrine pancreatic ducts. (D-F) Liver. WNK3 localizes to intercellular junctions of epithelial cells that line bile ducts. (G-I) Small intestine. WNK3 is in enterocytes in the crypts of Lieberkuhn. (J-L) Stomach. WNK3 is at intercellular junctions of HCl-secreting epithelial cells lining gastric glands. (Original magnification: ×630.)
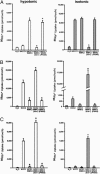
Fig. 3.
WNK3 has opposing effects on NKCC1 and KCC1/KCC2. Xenopus oocytes were injected with cRNAs encoding either NKCC1 (A), KCC2 (B), or KCC1 (C) alone or in combination with wild-type or mutant WNK3. NKCC1-dependent 86Rb+ influx (A) or efflux (B and C) was determined as described in Methods. Data show the mean ± SE of 86Rb+ flux and are representative of at least four independent experiments. (A) WNK3 regulates NKCC1. NKCC1 normally shows negligible activity under hypotonic conditions (180 mosM) and is partially active in isotonic conditions (200 mM). WNK3 increases NKCC1 to maximal activity in both conditions. Conversely, kinase-dead WNK3 strongly inhibits NKCC1 activity. *, P < 0.0001 vs. NKCC1 alone. (B) WNK3 regulates KCC2. KCC2 alone is partially active under isotonic conditions and induced under hypotonic conditions. WNK3 inhibits KCC2 under both conditions. In contrast, kinase-dead WNK3 strongly activates KCC2 under both hypotonic and isotonic conditions. *, P < 0.0001 vs. KCC2 alone. (C) WNK3 regulates KCC1. KCC1 alone is inactive under isotonic conditions and induced under hypotonic conditions. WNK3 inhibits KCC1 under both conditions. In contrast, kinase-dead WNK3 strongly activates KCC1 under both hypotonic and isotonic conditions. *, P < 0.0001 vs. KCC1 alone.
Fig. 4.
WNK3 regulates the phosphorylation of NKCC1. Xenopus oocytes were injected with the indicated constructs and incubated at varying extracellular osmolarities. After incubation, oocytes were lysed, and Western blotting was performed by using the R5 (anti-Phospho-NKCC1) or T4 (anti-NKCC1) antibodies, as in Methods. Phosphorylation of NKCC1 normally increases from negligible levels in hypotonic conditions (180 mM) to complete phosphorylation in hypertonic conditions (220 mM). In contrast, coexpression of NKCC1 with kinase-active WNK3 results in robust phosphorylation of NKCC1 at all osmolarities. Conversely, expression of kinase-dead WNK3 results in marked reduction of NKCC1 phosphorylation.
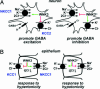
Fig. 5.
Proposed physiologic roles of WNK3. (A) WNK3 regulation of the neuronal response to GABA. Kinase-active WNK3 can increase [Cl-]i by increasing activity of NKCC1 and inhibiting KCC2, promoting an excitatory response to GABA. Kinase-dead WNK3 inhibits NKCC1 and activates KCC2, potentially promoting neruonal inhibition. (B) WNK3 regulation of cell volume. WNK3 can increase [Cl-]i by activating NKCC1 and inhibiting KCC1; kinase-inactive WNK3 could decrease [Cl-]i by inhibiting NKCC1 and activating KCC1. These are activities required to maintain cell volume in response to hyper- and hypoosmolar stress, respectively.
Similar articles
- WNK3 is a putative chloride-sensing kinase.
Pacheco-Alvarez D, Gamba G. Pacheco-Alvarez D, et al. Cell Physiol Biochem. 2011;28(6):1123-34. doi: 10.1159/000335848. Epub 2011 Dec 16. Cell Physiol Biochem. 2011. PMID: 22179001 Review. - WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway.
de Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vázquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G. de Los Heros P, et al. Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1976-81. doi: 10.1073/pnas.0510947103. Epub 2006 Jan 30. Proc Natl Acad Sci U S A. 2006. PMID: 16446421 Free PMC article. - WNK3 and WNK4 exhibit opposite sensitivity with respect to cell volume and intracellular chloride concentration.
Pacheco-Alvarez D, Carrillo-Pérez DL, Mercado A, Leyva-Ríos K, Moreno E, Hernández-Mercado E, Castañeda-Bueno M, Vázquez N, Gamba G. Pacheco-Alvarez D, et al. Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C371-C380. doi: 10.1152/ajpcell.00488.2019. Epub 2020 Jun 24. Am J Physiol Cell Physiol. 2020. PMID: 32579473 Free PMC article. - WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkalaemia, regulates the K+ channel ROMK1 (Kir1.1).
Leng Q, Kahle KT, Rinehart J, MacGregor GG, Wilson FH, Canessa CM, Lifton RP, Hebert SC. Leng Q, et al. J Physiol. 2006 Mar 1;571(Pt 2):275-86. doi: 10.1113/jphysiol.2005.102202. Epub 2005 Dec 15. J Physiol. 2006. PMID: 16357011 Free PMC article. - Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases.
Kahle KT, Rinehart J, Lifton RP. Kahle KT, et al. Biochim Biophys Acta. 2010 Dec;1802(12):1150-8. doi: 10.1016/j.bbadis.2010.07.009. Epub 2010 Jul 15. Biochim Biophys Acta. 2010. PMID: 20637866 Free PMC article. Review.
Cited by
- Regulation of brain tumor dispersal by NKCC1 through a novel role in focal adhesion regulation.
Garzon-Muvdi T, Schiapparelli P, ap Rhys C, Guerrero-Cazares H, Smith C, Kim DH, Kone L, Farber H, Lee DY, An SS, Levchenko A, Quiñones-Hinojosa A. Garzon-Muvdi T, et al. PLoS Biol. 2012;10(5):e1001320. doi: 10.1371/journal.pbio.1001320. Epub 2012 May 1. PLoS Biol. 2012. PMID: 22570591 Free PMC article. - Sites of regulated phosphorylation that control K-Cl cotransporter activity.
Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Rinehart J, et al. Cell. 2009 Aug 7;138(3):525-36. doi: 10.1016/j.cell.2009.05.031. Cell. 2009. PMID: 19665974 Free PMC article. - Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK).
Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Fraser SA, et al. Biochem J. 2007 Jul 1;405(1):85-93. doi: 10.1042/BJ20061850. Biochem J. 2007. PMID: 17341212 Free PMC article. - Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2.
Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C. Medina I, et al. Front Cell Neurosci. 2014 Feb 6;8:27. doi: 10.3389/fncel.2014.00027. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24567703 Free PMC article. Review. - WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis.
Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. Rinehart J, et al. Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16777-82. doi: 10.1073/pnas.0508303102. Epub 2005 Nov 7. Proc Natl Acad Sci U S A. 2005. PMID: 16275913 Free PMC article.
References
- Boron, W. F. & Boulpaep, E. L. (2005) Medical Physiology: A Cellular and Molecular Approach (Elsevier Saunders, Philadelphia).
- Lang, F., Busch, G. L., Ritter, M., Volkl, H., Waldegger, S., Gulbins, E. & Haussinger, D. (1998) Physiol. Rev. 78, 247-306. - PubMed
- Parker, J. (1994) in Cellular and Molecular Physiology of Cell Volume Regulation, ed. Strange, K. (CRC, Boca Raton, FL), pp. 311-321.
- Lytle, C. (1998) Am. J. Physiol. 274, 1002-1010. - PubMed
- Adragna, N. C., Fulvio, M. D. & Lauf, P. K. (2004) J. Membr. Biol. 201, 109-137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases