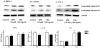Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells - PubMed (original) (raw)
Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells
Melinda C Myzak et al. Carcinogenesis. 2006 Apr.
Abstract
Sulforaphane (SFN), an isothiocyanate first isolated from broccoli, exhibits chemopreventive properties in prostate cancer cells through mechanisms that are poorly understood. We recently reported on a novel mechanism of chemoprotection by SFN in human colon cancer cells, namely the inhibition of histone deacetylase (HDAC). Here, we show that addition of 15 microM SFN also inhibited HDAC activity by 40, 30 and 40% in BPH-1, LnCaP and PC-3 prostate epithelial cells, respectively. The inhibition of HDAC was accompanied by a 50-100% increase in acetylated histones in all three prostate cell lines, and in BPH-1 cells treated with SFN there was enhanced interaction of acetylated histone H4 with the promoter region of the P21 gene and the bax gene. A corresponding 1.5- to 2-fold increase was seen for p21Cip1/Waf1 and Bax protein expression, consistent with previous studies using HDAC inhibitors, such as trichostatin A. The downstream events included cell cycle arrest and activation of apoptosis, as evidenced by changes in cell cycle kinetics and induction of multi-caspase activity. These findings provide new insight into the mechanisms of SFN action in benign prostate hyperplasia, androgen-dependent prostate cancer and androgen-independent prostate cancer cells, and they suggest a novel approach to chemoprotection and chemotherapy of prostate cancer through the inhibition of HDAC.
Conflict of interest statement
Conflict of Interest Statement: None declared.
Figures
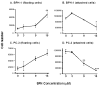
Fig. 1
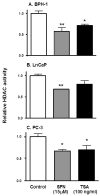
SFN inhibits HDAC activity in prostate epithelial cells. BPH-1 (A), LnCaP (B) and PC-3 cells (C) were harvested 48 h after treatment with SFN (15 μM), or 8 h after TSA exposure (100 ng/ml), and cell lysates were analyzed for HDAC activity, as reported (19). Results = normalized mean ± SD, n = 3. *P <0.05, **P <0.01. Average baseline HDAC activity for BPH-1, LnCaP and PC-3 cells were 7.7, 8.4 and 12.2 arbitrary florescence units (AFU), respectively.
Fig. 2
SFN increases acetylated histone levels in prostate cell lines. Cells were treated with SFN or TSA as described in the legend to Figure 1, and acetylated histone H3 or acetylated histone H4 levels were assessed by immunoblotting. Equal protein loading was confirmed using β-actin. Results are representative of two or more separate experiments. Fold change was calculated using density values normalized to β-actin and expressed as relative change between SFN/TSA treated and control.
Fig. 3
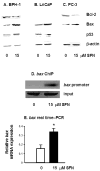
SFN increases p21 expression in prostate cells. (A), BPH-1 cells were treated with SFN and 48 h later DNA was cross-linked to proteins, ChIP was performed using acetylated histone H4, and following reversal of cross-linking and isolation of DNA, PCR was performed with primers to the P21 promoter. Wedge symbol, 3, 9, 15 μM SFN; control, 0 μM SFN (vehicle alone). (B), Cells were treated with 0 or 15 μM SFN and 48 h later the attached cells were isolated and cell lysates were immunoblotted for p21 protein. Equal protein loading was confirmed using β-actin. Results are representative of two or more separate experiments. Fold change was calculated using density values normalized to β-actin and expressed as relative change between 0 and 15 μM SFN.
Fig. 4
SFN alters the expression of pro- and anti-apoptotic proteins. Attached cells were harvested 48 h after treatment of BPH-1 (A), LnCaP (B) and PC-3 cells (C) with 0 or 15 μM SFN, and cell lysates were immunoblotted for Bcl-2, Bax and p53, as indicated. Equal protein loading was confirmed using β-actin. Results are representative of two or more separate experiments. (D) BPH-1 cells were treated with 0 or 15 μM SFN and ChIP was performed as described in the legend to Figure 3, except that PCR was performed with primers to the bax promoter. (E) Real-time PCR results for bax mRNA expression in BPH-1 cells 12 h after treatment with 0 or 15 μM SFN; mean ± SD, n = 3; *P <0.05.
Fig. 5
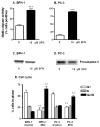
SFN increases multi-caspase activity and causes cell cycle arrest in prostate epithelial cells. BPH-1 cells (A) and PC-3 cells (B) were harvested 48 h after treatment with 0 or 15 μM SFN, as indicated, and the attached cells were examined for multi-caspase activity using a Guava PCA. Results represent mean ± SD, n = 3, from experiments conducted on two or more separate occasions. BPH-1 (C) and PC-3 (D) cells were treated as above, and cell lysates were immunoblotted for procaspase-3. In (E) BPH-1 and PC-3 were harvested, fixed and stained for cell cycle analysis. Attached and floating cells were fixed in 70% ethanol and stained with propidium iodide, and cell cycle kinetics was examined using the Guava PCA, followed by data analysis with Multi-Cycle software. Results indicate mean ± SD, n = 3; **P <0.01, ***P <0.001.
Fig. 6
SFN increases the proportion of prostate cells in the floating population. BPH-1 (A and B) and PC-3 cells (C and D) were treated with 0, 3, 9, or 15 μM SFN and 48 h later the floating and attached cells were counted. Results are given as mean ± SD, n = 3. *P <0.05, **P <0.01, ***P <0.001.
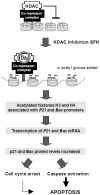
Fig. 7
Model for the mechanism of SFN-induced apoptosis through HDAC inhibition in prostate cells. SFN inhibits HDAC activity, which results in an increase in acetylated histones associated with the promoter region of genes such as P21 and bax. Interactions between the histones and DNA are loosened, facilitating transcription factor access to P21 and bax. The corresponding de-repression of these genes activates transcription, leading to increased mRNA and protein expression, enabling cycle arrest, caspase activation, and apoptosis induction in prostate cancer cells.
Similar articles
- Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells.
Clarke JD, Hsu A, Yu Z, Dashwood RH, Ho E. Clarke JD, et al. Mol Nutr Food Res. 2011 Jul;55(7):999-1009. doi: 10.1002/mnfr.201000547. Epub 2011 Mar 4. Mol Nutr Food Res. 2011. PMID: 21374800 Free PMC article. - A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase.
Myzak MC, Karplus PA, Chung FL, Dashwood RH. Myzak MC, et al. Cancer Res. 2004 Aug 15;64(16):5767-74. doi: 10.1158/0008-5472.CAN-04-1326. Cancer Res. 2004. PMID: 15313918 - Histone deacetylase turnover and recovery in sulforaphane-treated colon cancer cells: competing actions of 14-3-3 and Pin1 in HDAC3/SMRT corepressor complex dissociation/reassembly.
Rajendran P, Delage B, Dashwood WM, Yu TW, Wuth B, Williams DE, Ho E, Dashwood RH. Rajendran P, et al. Mol Cancer. 2011 May 30;10:68. doi: 10.1186/1476-4598-10-68. Mol Cancer. 2011. PMID: 21624135 Free PMC article. - Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds.
Nian H, Delage B, Ho E, Dashwood RH. Nian H, et al. Environ Mol Mutagen. 2009 Apr;50(3):213-21. doi: 10.1002/em.20454. Environ Mol Mutagen. 2009. PMID: 19197985 Free PMC article. Review. - Multi-targeted prevention of cancer by sulforaphane.
Clarke JD, Dashwood RH, Ho E. Clarke JD, et al. Cancer Lett. 2008 Oct 8;269(2):291-304. doi: 10.1016/j.canlet.2008.04.018. Epub 2008 May 27. Cancer Lett. 2008. PMID: 18504070 Free PMC article. Review.
Cited by
- Molecular Pathways Related to Sulforaphane as Adjuvant Treatment: A Nanomedicine Perspective in Breast Cancer.
Saavedra-Leos MZ, Jordan-Alejandre E, Puente-Rivera J, Silva-Cázares MB. Saavedra-Leos MZ, et al. Medicina (Kaunas). 2022 Oct 1;58(10):1377. doi: 10.3390/medicina58101377. Medicina (Kaunas). 2022. PMID: 36295538 Free PMC article. Review. - Sulforaphane improves chemotherapy efficacy by targeting cancer stem cell-like properties via the miR-124/IL-6R/STAT3 axis.
Wang X, Li Y, Dai Y, Liu Q, Ning S, Liu J, Shen Z, Zhu D, Jiang F, Zhang J, Li Z. Wang X, et al. Sci Rep. 2016 Nov 8;6:36796. doi: 10.1038/srep36796. Sci Rep. 2016. PMID: 27824145 Free PMC article. - Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis.
Higdon JV, Delage B, Williams DE, Dashwood RH. Higdon JV, et al. Pharmacol Res. 2007 Mar;55(3):224-36. doi: 10.1016/j.phrs.2007.01.009. Epub 2007 Jan 25. Pharmacol Res. 2007. PMID: 17317210 Free PMC article. Review. - Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention.
Ho E, Clarke JD, Dashwood RH. Ho E, et al. J Nutr. 2009 Dec;139(12):2393-6. doi: 10.3945/jn.109.113332. Epub 2009 Oct 7. J Nutr. 2009. PMID: 19812222 Free PMC article. - Transforming growth factor-β and nuclear factor E2–related factor 2 regulate antioxidant responses in airway smooth muscle cells: role in asthma.
Michaeloudes C, Chang PJ, Petrou M, Chung KF. Michaeloudes C, et al. Am J Respir Crit Care Med. 2011 Oct 15;184(8):894-903. doi: 10.1164/rccm.201011-1780OC. Am J Respir Crit Care Med. 2011. PMID: 21799075 Free PMC article.
References
- Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. - PubMed
- Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. - PubMed
- Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. - PubMed
- Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of cruciferous vegetables and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1403–1409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA090890/CA/NCI NIH HHS/United States
- P01 CA090890-01A20003/CA/NCI NIH HHS/United States
- R01 CA080176/CA/NCI NIH HHS/United States
- CA90890/CA/NCI NIH HHS/United States
- CA80176/CA/NCI NIH HHS/United States
- CA107693/CA/NCI NIH HHS/United States
- P01 CA090890-05/CA/NCI NIH HHS/United States
- R01 CA065525/CA/NCI NIH HHS/United States
- R01 CA065525-08/CA/NCI NIH HHS/United States
- R01 CA107693/CA/NCI NIH HHS/United States
- R01 CA065525-09/CA/NCI NIH HHS/United States
- CA66525/CA/NCI NIH HHS/United States
- R01 CA080176-05/CA/NCI NIH HHS/United States
- P01 CA090890-01A29001/CA/NCI NIH HHS/United States
- P30 ES000210/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous