Acute oxygen-sensing mechanisms - PubMed (original) (raw)
Review
Acute oxygen-sensing mechanisms
E Kenneth Weir et al. N Engl J Med. 2005.
No abstract available
Figures
Figure 1. Homeostatic Oxygen-Sensing System
Specialized tissues that sense the local oxygen level are shown. The carotid body at the carotid-artery bifurcation increases action-potential frequency in the carotid-sinus nerve in response to hypoxia, thus stimulating respiration. The small resistance pulmonary and fetoplacental arteries demonstrate hypoxic vasoconstriction, optimizing oxygen transfer in the lung and placenta. The ductus arteriosus, by contrast, contracts when oxygen levels rise, redirecting blood through the newly expanded lungs of the newborn. The neuroepithelial bodies in the lungs and adrenomedullary cells in the fetus also sense oxygen.
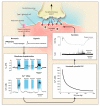
Figure 2. Opposite Regulation of Potassium Channels by Oxygen in Pulmonary-Artery as Compared with Ductus Smooth-Muscle Cells
In the pulmonary-artery smooth-muscle cell (shown in the upper half of the figure) during normoxia, an outward potassium (K+) current, illustrated by the single channel trace that shows steplike opening and closing, keeps the membrane potential at about −50 mV or −60 mV. This hyperpolarization prevents calcium from entering the cell through the voltage-gated L-type calcium channel. Hypoxia inhibits potassium-channel activity and depolarizes the membrane to about −20 mV, permitting calcium entry. In the ductus smooth-muscle cell (lower half of the figure), by contrast, the outward potassium current is maintained during hypoxia and is inhibited by normoxia. A rise in oxygen, as at birth, then causes membrane depolarization and calcium entry.
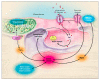
Figure 3. Oxygen Sensing in the Carotid Body
The chief function of the carotid body is to increase respiration in response to hypoxia. The proximal pathway in the type 1 cell of the carotid body is similar to that in the pulmonary-artery smooth-muscle cell. Hypoxia inhibits potassium-channel activity, shown in the single channel trace, causing membrane depolarization, calcium influx, secretion, and increased action potentials in the carotid-sinus nerve. If the membrane potential (Em) is “clamped” at −60 mV, hypoxia no longer leads to an increase in the cytosolic calcium (Ca2+i), indicating that the increase in calcium requires membrane depolarization. Cytosolic calcium normally rises sharply as oxygen levels fall below 60 mm Hg. Increased calcium stimulates the release of dopamine, a marker for secretion. pA denotes picoamperes.
Figure 4. Redox Mechanism for Oxygen Sensing in Specialized Tissues
Reactive oxygen species (ROS) from the mitochondria, NADPH oxidase, NADH oxidase, or redox couples may control potassium-channel gating and membrane potential (Em) and thus calcium entry. The same redox signaling may control calcium release from the sarcoplasmic reticulum. The calcium stores in the sarcoplasmic reticulum, in turn, are repleted by calcium entry through the store-operated channels. Rho kinase augments the response of actin–myosin at any level of cytosolic calcium (Ca2+i). SOD denotes superoxide dismutase, H2O2 hydrogen peroxide, GSH glutathione, and GSSG oxidized glutathione.
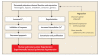
Figure 5. Effects of Decreased Potassium-Channel Function or Expression in Pulmonary-Artery Hypertension
Decreased function, expression, or both, of potassium channels, initiated by a variety of agents, can lead to vasoconstriction, proliferation, and decreased apoptosis. Consequently, regression of pulmonary hypertension may be achieved by opening potassium channels or increasing potassium-channel expression. Other mechanisms involving the endothelium are not illustrated. K+i denotes cytosolic potassium concentration. Up arrow denotes enhanced expression.
Comment in
- Acute oxygen-sensing mechanisms.
Moskowitz DW. Moskowitz DW. N Engl J Med. 2006 Mar 2;354(9):975-7; author reply 975-7. doi: 10.1056/NEJMc053396. N Engl J Med. 2006. PMID: 16510756 No abstract available. - Acute oxygen-sensing mechanisms.
Eltzschig HK, Karhausen J, Kempf VA. Eltzschig HK, et al. N Engl J Med. 2006 Mar 2;354(9):975-7; author reply 975-7. N Engl J Med. 2006. PMID: 16514707 No abstract available. - Acute oxygen-sensing mechanisms.
Nauseef WM. Nauseef WM. N Engl J Med. 2006 Mar 2;354(9):975-7; author reply 975-7. N Engl J Med. 2006. PMID: 16514708 No abstract available. - Acute oxygen-sensing mechanisms.
Khan S. Khan S. N Engl J Med. 2006 Mar 2;354(9):975-7; author reply 975-7. N Engl J Med. 2006. PMID: 16514709 No abstract available.
Similar articles
- Mechanisms of oxygen sensing: a key to therapy of pulmonary hypertension and patent ductus arteriosus.
Weir EK, Obreztchikova M, Vargese A, Cabrera JA, Peterson DA, Hong Z. Weir EK, et al. Br J Pharmacol. 2008 Oct;155(3):300-7. doi: 10.1038/bjp.2008.291. Epub 2008 Jul 21. Br J Pharmacol. 2008. PMID: 18641675 Free PMC article. Review. - Pulmonary vasoconstriction, oxygen sensing, and the role of ion channels: Thomas A. Neff lecture.
Weir EK, Reeve HL, Peterson DA, Michelakis ED, Nelson DP, Archer SL. Weir EK, et al. Chest. 1998 Jul;114(1 Suppl):17S-22S. doi: 10.1378/chest.114.1_supplement.17s-a. Chest. 1998. PMID: 9676606 Review. No abstract available. - An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension.
Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. Bonnet S, et al. Circulation. 2006 Jun 6;113(22):2630-41. doi: 10.1161/CIRCULATIONAHA.105.609008. Epub 2006 May 30. Circulation. 2006. PMID: 16735674 - Hypoxic pulmonary vasoconstriction.
Moudgil R, Michelakis ED, Archer SL. Moudgil R, et al. J Appl Physiol (1985). 2005 Jan;98(1):390-403. doi: 10.1152/japplphysiol.00733.2004. J Appl Physiol (1985). 2005. PMID: 15591309 Review. - Acute hypoxic pulmonary vasoconstriction: a model of oxygen sensing.
Michelakis ED, Archer SL, Weir EK. Michelakis ED, et al. Physiol Res. 1995;44(6):361-7. Physiol Res. 1995. PMID: 8798271 Review.
Cited by
- Hypoxia-dependent signaling in perioperative and critical care medicine.
Hirota K. Hirota K. J Anesth. 2021 Oct;35(5):741-756. doi: 10.1007/s00540-021-02940-w. Epub 2021 May 18. J Anesth. 2021. PMID: 34003375 Free PMC article. Review. - Pulmonary hypertension.
Mocumbi A, Humbert M, Saxena A, Jing ZC, Sliwa K, Thienemann F, Archer SL, Stewart S. Mocumbi A, et al. Nat Rev Dis Primers. 2024 Jan 4;10(1):1. doi: 10.1038/s41572-023-00486-7. Nat Rev Dis Primers. 2024. PMID: 38177157 Review. - TRP channels: sensors and transducers of gasotransmitter signals.
Takahashi N, Kozai D, Mori Y. Takahashi N, et al. Front Physiol. 2012 Aug 9;3:324. doi: 10.3389/fphys.2012.00324. eCollection 2012. Front Physiol. 2012. PMID: 22934072 Free PMC article. - Pheochromocytoma complicated by cyanotic congenital heart disease: a case report.
Yamamoto K, Namba N, Kubota T, Usui T, Takahashi K, Kitaoka T, Fujiwara M, Hori Y, Kogaki S, Oue T, Morii E, Ozono K. Yamamoto K, et al. Clin Pediatr Endocrinol. 2016 Apr;25(2):59-65. doi: 10.1297/cpe.25.59. Epub 2016 Apr 28. Clin Pediatr Endocrinol. 2016. PMID: 27212797 Free PMC article. - Role of mitochondrial mutations in cancer.
Baysal BE. Baysal BE. Endocr Pathol. 2006 Fall;17(3):203-12. doi: 10.1385/ep:17:3:203. Endocr Pathol. 2006. PMID: 17308357 Review.
References
- Priestly J. Experiments and observations on different kinds of air. 2nd ed Vol. 1775. J. Johnson; London: p. 101.
- Hales CA, Westphal D. Hypoxemia following the administration of sublingual nitroglycerin. Am J Med. 1978;65:911–8. - PubMed
- Motley H, Cournard A, Werko L, Himmelstein A, Dresdale D. The influence of short periods of induced acute anoxia upon pulmonary artery pressures in man. Am J Physiol. 1947;150:315–20. - PubMed
- Hambraeus-Jonzon K, Bindslev L, Mellgard A, Hedenstierna G. Hypoxic pulmonary vasoconstriction in human lungs: a stimulus-response study. Anesthesiology. 1997;86:308–15. - PubMed
- Dorrington KL, Clar C, Young JD, Jonas M, Tansley JG, Robbins PA. Time course of the human pulmonary vascular response to 8 hours of isocapnic hypoxia. Am J Physiol. 1997;273:H1126–H1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL-65322/HL/NHLBI NIH HHS/United States
- R01-HL071115/HL/NHLBI NIH HHS/United States
- R01 HL071115/HL/NHLBI NIH HHS/United States
- R01 HL065322/HL/NHLBI NIH HHS/United States
- R01 HL065322-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources