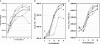Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice - PubMed (original) (raw)
Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice
Boyd Yount et al. J Virol. 2005 Dec.
Abstract
SARS coronavirus (SARS-CoV) encodes several unique group-specific open reading frames (ORFs) relative to other known coronaviruses. To determine the significance of the SARS-CoV group-specific ORFs in virus replication in vitro and in mice, we systematically deleted five of the eight group-specific ORFs, ORF3a, OF3b, ORF6, ORF7a, and ORF7b, and characterized recombinant virus replication and gene expression in vitro. Deletion of the group-specific ORFs of SARS-CoV, either alone or in various combinations, did not dramatically influence replication efficiency in cell culture or in the levels of viral RNA synthesis. The greatest reduction in virus growth was noted following ORF3a deletion. SARS-CoV spike (S) glycoprotein does not encode a rough endoplasmic reticulum (rER)/Golgi retention signal, and it has been suggested that ORF3a interacts with and targets S glycoprotein retention in the rER/Golgi apparatus. Deletion of ORF3a did not alter subcellular localization of the S glycoprotein from distinct punctuate localization in the rER/Golgi apparatus. These data suggest that ORF3a plays little role in the targeting of S localization in the rER/Golgi apparatus. In addition, insertion of the 29-bp deletion fusing ORF8a/b into the single ORF8, noted in early-stage SARS-CoV human and civet cat isolates, had little if any impact on in vitro growth or RNA synthesis. All recombinant viruses replicated to wild-type levels in the murine model, suggesting that either the group-specific ORFs play little role in in vivo replication efficiency or that the mouse model is not of sufficient quality for discerning the role of the group-specific ORFs in disease origin and development.
Figures
FIG. 1.
Genome organization of SARS-CoV recombinant viruses. The typical genome organization of the SARS-CoV includes a ∼21-kb replicase encoded in ORF1a and ORF1b; the structural genes S (ORF2), E (ORF4), M (ORF5), and N (ORF9); and the group-specific ORFs 3a (X1), 3b (X2), 6(X3), 7a(X4)/b, 8a/b(X5), and 9b. Among SARS-CoV isolates obtained from animals or early-phase human cases, ORF8a/b is fused into a single contiguous ORF8 by the presence of 29 nucleotides that are deleted in middle/late-phase epidemic strains like Urbani and Tor-2. As described in the Materials and Methods, various SARS-CoV group-specific ORFs were deleted, either individually or in combinations. The icSARS-CoV ΔORF3a lacks ORF3a, icSARS-CoV ΔORF6 lacks ORF6, and icSARS-CoV ΔORF7a/b lacks ORF7a/b, while icSARS-CoV ΔORF3a/b and icSARS-CoV ΔORF3&6 lack ORF3a/b and ORF3a/b/ORF6, respectively. The recombinant virus icSARS-CoV SZ16 ORF8 contains a full-length ORF8, produced by the insertion of the 29 nt that are present in animal and early-phase human cases that fuses ORF8a and-8b into a single ORF. Recombinant cDNAs containing the group-specific ORF deletions or insertions were confirmed by sequence analysis and used to assemble full-length cDNAs for the recovery of recombinant SARS-CoV. TRS, transcription regulatory sequence.
FIG.2.
Genotype characterization of recombinant viruses. Cultures of Vero cells were electroporated with full-length transcripts derived from different recombinant full-length cDNAs. Virus progeny were harvested between 24 and 36 h posttransfection and plaque purified and stocks generated for future studies. Cultures of cells were infected with stock viruses and intracellular RNA harvested at 12 h postinfection. Using various primer pairs that span the deletions, recombinant viruses were genotyped by RT-PCR (panels A and B). Primer location and expected sizes of PCR amplicons are shown in panel C. Primer pairs #44, #45, and #46 and SARS CoV E(−) were used in panel A, while primer pairs #47 and 48 and SARS-CoV ORF8b(−) were used in panel B. These data confirm that the deletions inserted into the component clones were found in the appropriate recombinant viruses. WT, wild type.
FIG.2.
Genotype characterization of recombinant viruses. Cultures of Vero cells were electroporated with full-length transcripts derived from different recombinant full-length cDNAs. Virus progeny were harvested between 24 and 36 h posttransfection and plaque purified and stocks generated for future studies. Cultures of cells were infected with stock viruses and intracellular RNA harvested at 12 h postinfection. Using various primer pairs that span the deletions, recombinant viruses were genotyped by RT-PCR (panels A and B). Primer location and expected sizes of PCR amplicons are shown in panel C. Primer pairs #44, #45, and #46 and SARS CoV E(−) were used in panel A, while primer pairs #47 and 48 and SARS-CoV ORF8b(−) were used in panel B. These data confirm that the deletions inserted into the component clones were found in the appropriate recombinant viruses. WT, wild type.
FIG. 3.
Recombinant virus growth kinetics. Cultures of Vero, MA104, or human CACO2 cells were inoculated with various recombinant or wild-type viruses at an MOI of 1.0. Samples were taken at the indicated times and virus titers determined by plaque assay in Vero cells. (A) Growth in Vero cells; (B) growth in MA104 cells; (C) growth in CACO2 cells. Symbols: •, Urbani SARS-CoV; ○, icSARS-CoV; □, icSARS-CoV ΔORF3a; ▪, icSARS-CoV ΔORF3; ▵, icSARS-CoV ΔORF6; ▴, icSARS-CoV ΔORF7; ⧫, icSARS-CoV ΔORF3&6.
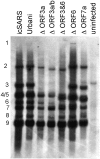
FIG. 4.
Recombinant virus RNA synthesis. Cultures of Vero cells were infected with various recombinant or wild-type viruses at an MOI of 1.0. Intracellular RNA was isolated as described in the Materials and Methods and separated on 1% agarose gels. The RNA was transferred to a BrightStar-Plus membrane (Ambion) for 4 to 5 h and the RNA cross-linked to the membrane by UV light. The blot was probed with an N-gene-specific oligodeoxynucleotide probe (5′-CTTGACTGCCGCCTCTGCTbTbCCCTbCTbGCb-3′), where biontinylated nucleotides are designated with a superscript b. Blots were hybridized overnight and washed with low- and high-stringency buffers as recommended by the manufacturer (BrightStar BioDetect chemiluminescent detection system; Ambion). Filters were overlaid with film and developed.
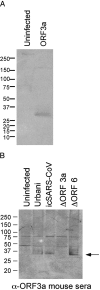
FIG. 5.
Expression of ORF3a in VRP and recombinant viruses. Vero cells were infected with VRPs encoding the SARS ORF3a (VRP-ORF3a) (A) or with various recombinant or wild-type SARS-CoV (B). The proteins were harvested at 12 h postinfection, separated on polyacrylamide gels, and probed with convalescent human antiserum #1128 from a SARS-CoV-infected patient (A) or antiserum raised from mice inoculated with VRP-ORF3a (B).
FIG.6.
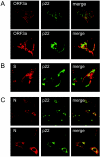
Colocalization of SARS-CoV spike, ORF3a, and nucleocapsid proteins with replicase protein. Cultures of Vero cells were inoculated with wild-type Urbani SARS-CoV and fixed at 6 and 9 h postinfection as described in the Materials and Methods. Antisera against the SARS-CoV S glycoprotein, nucleocapsid, and ORF3a protein were used to determine cell distributions in combination with the SARS-CoV replicase protein, p22, by confocal microscopy as previously described (45). (A) Colocalization and distribution of the SARS-CoV ORF3a and p22 replicase protein at 6 h postinfection. (B) Colocalization and distribution of the SARS-CoV S glycoprotein and p22 replicase protein at 6 h postinfection. (C) Colocalization and distribution of the SARS-CoV nucleocapsid protein and p22 replicase protein at 6 h (top row) and 9 h (bottom row) postinfection.
FIG. 7.
Subcellular distribution of S glycoprotein in group-specific ORF deletion viruses. Cultures of Vero cells were inoculated with wild-type Urbani SARS-CoV, icSARS-CoV, or icSARS-CoV ΔORF3a. At 12 h, the infected cells were fixed in ice-cold 100% methanol overnight, washed with PBS, and blocked in PBS containing 5% BSA. Cells were washed and the coverslips incubated with mouse anti-S or ORF3a antibody diluted 1:1,000 in PBS containing 1% BSA, 0.05% NP-40, and 2% normal goat serum. Secondary antibody was fluorescein isothiocyanate-conjugated anti-mouse antibody diluted 1:1,000 in PBS containing 1% BSA, 0.05% NP-40, and 2% normal goat serum. Coverslips were washed, mounted, and visualized with a Nikon Microphot FXA Upright fluorescence microscope.
FIG. 8.
In vivo replication of recombinant SARS-CoV. Six-week-old BALB/c mice were inoculated intranasally with 50 μl inoculate containing 2 × 105 PFU of wild-type and various recombinant viruses. At 2 days postinfection, the animals were sacrificed and virus titers in the lungs determined by plaque assay (A). To determine if an intact ORF8 alters in vivo pathogenesis in the murine model, a recombinant virus was constructed which included the 29 bp that fuse ORF8a/b into a single contiguous ORF. The recombinant icSARS-CoV civet SZ16 ORF8 virus replicated as efficiently as wild-type virus in Vero cells (B), contained the appropriate 29-bp insertion by RT-PCR genotyping (C), and replicated to similar titers as wild-type virus in BALB/c mice (A). Symbols: •, Urbani SARS-CoV; ○, icSARS-CoV; □, icSARS-CoV SZ16 ORF8.
Similar articles
- Understanding the accessory viral proteins unique to the severe acute respiratory syndrome (SARS) coronavirus.
Tan YJ, Lim SG, Hong W. Tan YJ, et al. Antiviral Res. 2006 Nov;72(2):78-88. doi: 10.1016/j.antiviral.2006.05.010. Epub 2006 Jun 6. Antiviral Res. 2006. PMID: 16820226 Free PMC article. Review. - A severe acute respiratory syndrome-associated coronavirus-specific protein enhances virulence of an attenuated murine coronavirus.
Pewe L, Zhou H, Netland J, Tangudu C, Olivares H, Shi L, Look D, Gallagher T, Perlman S. Pewe L, et al. J Virol. 2005 Sep;79(17):11335-42. doi: 10.1128/JVI.79.17.11335-11342.2005. J Virol. 2005. PMID: 16103185 Free PMC article. - Contribution of SARS-CoV-2 Accessory Proteins to Viral Pathogenicity in K18 Human ACE2 Transgenic Mice.
Silvas JA, Vasquez DM, Park JG, Chiem K, Allué-Guardia A, Garcia-Vilanova A, Platt RN, Miorin L, Kehrer T, Cupic A, Gonzalez-Reiche AS, Bakel HV, García-Sastre A, Anderson T, Torrelles JB, Ye C, Martinez-Sobrido L. Silvas JA, et al. J Virol. 2021 Aug 10;95(17):e0040221. doi: 10.1128/JVI.00402-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133899 Free PMC article. - Severe Acute Respiratory Syndrome Coronavirus ORF7a Inhibits Bone Marrow Stromal Antigen 2 Virion Tethering through a Novel Mechanism of Glycosylation Interference.
Taylor JK, Coleman CM, Postel S, Sisk JM, Bernbaum JG, Venkataraman T, Sundberg EJ, Frieman MB. Taylor JK, et al. J Virol. 2015 Dec;89(23):11820-33. doi: 10.1128/JVI.02274-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378163 Free PMC article. - Characterization of viral proteins encoded by the SARS-coronavirus genome.
Tan YJ, Lim SG, Hong W. Tan YJ, et al. Antiviral Res. 2005 Feb;65(2):69-78. doi: 10.1016/j.antiviral.2004.10.001. Antiviral Res. 2005. PMID: 15708633 Free PMC article. Review.
Cited by
- The Emerging Roles of Viroporins in ER Stress Response and Autophagy Induction during Virus Infection.
Fung TS, Torres J, Liu DX. Fung TS, et al. Viruses. 2015 Jun 4;7(6):2834-57. doi: 10.3390/v7062749. Viruses. 2015. PMID: 26053926 Free PMC article. Review. - Release of severe acute respiratory syndrome coronavirus nuclear import block enhances host transcription in human lung cells.
Sims AC, Tilton SC, Menachery VD, Gralinski LE, Schäfer A, Matzke MM, Webb-Robertson BJ, Chang J, Luna ML, Long CE, Shukla AK, Bankhead AR 3rd, Burkett SE, Zornetzer G, Tseng CT, Metz TO, Pickles R, McWeeney S, Smith RD, Katze MG, Waters KM, Baric RS. Sims AC, et al. J Virol. 2013 Apr;87(7):3885-902. doi: 10.1128/JVI.02520-12. Epub 2013 Jan 30. J Virol. 2013. PMID: 23365422 Free PMC article. - Impairment of antiviral immune response and disruption of cellular functions by SARS-CoV-2 ORF7a and ORF7b.
García-García T, Fernández-Rodríguez R, Redondo N, de Lucas-Rius A, Zaldívar-López S, López-Ayllón BD, Suárez-Cárdenas JM, Jiménez-Marín Á, Montoya M, Garrido JJ. García-García T, et al. iScience. 2022 Nov 18;25(11):105444. doi: 10.1016/j.isci.2022.105444. Epub 2022 Oct 25. iScience. 2022. PMID: 36310646 Free PMC article. - SARS-CoV-2 ORF8 and SARS-CoV ORF8ab: Genomic Divergence and Functional Convergence.
Mohammad S, Bouchama A, Mohammad Alharbi B, Rashid M, Saleem Khatlani T, Gaber NS, Malik SS. Mohammad S, et al. Pathogens. 2020 Aug 20;9(9):677. doi: 10.3390/pathogens9090677. Pathogens. 2020. PMID: 32825438 Free PMC article. Review. - The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8.
Oostra M, de Haan CA, Rottier PJ. Oostra M, et al. J Virol. 2007 Dec;81(24):13876-88. doi: 10.1128/JVI.01631-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928347 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI059443/AI/NIAID NIH HHS/United States
- R01 AI059136/AI/NIAID NIH HHS/United States
- AI059136/AI/NIAID NIH HHS/United States
- P01 AI059443-01A1/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous