Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells - PubMed (original) (raw)
Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells
M C W Chan et al. Respir Res. 2005.
Abstract
Background: Fatal human respiratory disease associated with influenza A subtype H5N1 has been documented in Hong Kong, and more recently in Vietnam, Thailand and Cambodia. We previously demonstrated that patients with H5N1 disease had unusually high serum levels of IP-10 (interferon-gamma-inducible protein-10). Furthermore, when compared with human influenza virus subtype H1N1, the H5N1 viruses in 1997 (A/Hong Kong/483/97) (H5N1/97) were more potent inducers of pro-inflammatory cytokines (e.g. tumor necrosis factor-a) and chemokines (e.g. IP-10) from primary human macrophages in vitro, which suggests that cytokines dysregulation may play a role in pathogenesis of H5N1 disease. Since respiratory epithelial cells are the primary target cell for replication of influenza viruses, it is pertinent to investigate the cytokine induction profile of H5N1 viruses in these cells.
Methods: We used quantitative RT-PCR and ELISA to compare the profile of cytokine and chemokine gene expression induced by H5N1 viruses A/HK/483/97 (H5N1/97), A/Vietnam/1194/04 and A/Vietnam/3046/04 (both H5N1/04) with that of human H1N1 virus in human primary alveolar and bronchial epithelial cells in vitro.
Results: We demonstrated that in comparison to human H1N1 viruses, H5N1/97 and H5N1/04 viruses were more potent inducers of IP-10, interferon beta, RANTES (regulated on activation, normal T cell expressed and secreted) and interleukin 6 (IL-6) in primary human alveolar and bronchial epithelial cells in vitro. Recent H5N1 viruses from Vietnam (H5N1/04) appeared to be even more potent at inducing IP-10 than H5N1/97 virus.
Conclusion: The H5N1/97 and H5N1/04 subtype influenza A viruses are more potent inducers of proinflammatory cytokines and chemokines in primary human respiratory epithelial cells than subtype H1N1 virus. We suggest that this hyper-induction of cytokines may be relevant to the pathogenesis of human H5N1 disease.
Figures
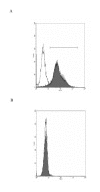
Figure 1
(A) Primary human type II pneumocytes were stained with antibody surfactant protein-C (shaded curve) and control antibody (unshaded curve) to confirm their identity. (B) Human type II pneumocytes isolated were stained with anti-CD14 FITC-conjugated antibodies (shaded curve) specific for macrophage surface antigen to check for any contaminant macrophage.
Figure 2
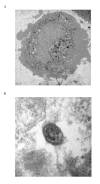
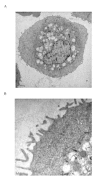
Transmission electron micrographs of human type II pneumocytes cultured in vitro (A) and the lamellar bodies in the cytoplasm demonstrated using higher magnification (B) (Bars: 1 μm and 50 nm respectively). The cells were scraped off the culture flask, fixed in 2% glutaraldehyde and embedded in Araldite resin.
Figure 4
Infection of human type II pneumocytes with human influenza viruses. (A) Purified alveolar epithelial cells were fixed and analyzed by immunofluorescent staining specific for influenza virus nucleoprotein (×150). (B) The influenza M-gene mRNA profiles were assayed after infection. The concentrations of M-gene mRNA were normalized to those of β-actin mRNA in the corresponding sample. Means of duplicate assays are shown. (C) Alveolar epithelial cells were infected with human influenza viruses and the infectious virus yield (log10TCID50/ml) was determined in aliquots of supernatant collected at various times. Data are the means and the standard errors of independent experiments from three separate donors.
Figure 5
Cytokine and chemokine gene expression profile of influenza-virus-infected human type II pneumocytes by quantitative RT-PCR. Cytokine and chemokine mRNA concentration were assayed 3 h and 6 h after infection with A/Hong Kong/483/97 (H5N1/97), A/Vietnam/1194/04, A/Vietnam/3046/04 (both H5N1/04) and A/Hong Kong 54/98 (H1N1) influenza viruses or in mock infected cells. H5N1/97 and both H5N1/04 influenza viruses induced significantly higher levels of IP-10, interferon-beta, RANTES and IL-6 when compared to H1N1 infected cells at 6 hours post-infection (p < 0.001, Bonferroni multiple comparison test). The mRNA concentrations of cytokine and chemokine mRNA were normalized to those β-actin mRNA in the corresponding samples. Means and standard deviation from experiments from five different donors are shown
Figure 3
Transmission electron micrographs of human bronchial epithelial cells in vitro at low (A) and high (B) magnification (Bars: 2 μm and 0.5 μm respectively). The cells were scraped off the culture flask, fixed in 2% glutaraldehyde and embedded in Araldite resin.
Figure 6
Infection of human bronchial epithelial cells with human influenza viruses. (A) The influenza M-gene mRNA profiles were assayed after infection. The concentrations of M-gene mRNA were normalized to those of β-actin mRNA in the corresponding sample. Means of duplicate assays are shown. (B) Virus yields (log10TCID50/ml) were determined in aliquots of supernatant collected from influenza-infected bronchial epithelial cells at various times. Data are the means and the standard errors of two independent experiments.
Figure 7
Cytokine and chemokine gene expression profile of influenza-virus-infected human bronchial epithelial cells by quantitative RT-PCR. Cytokine and chemokine mRNA concentration were assayed 3 h and 6 h after infection with A/Hong Kong/483/97 (H5N1/97), A/Vietnam/1194/04, A/Vietnam/3046/04 (both H5N1/04) and A/Hong Kong 54/98 (H1N1) influenza viruses or in mock infected cells. When compared with H1N1 infected cells, H5N1/97 and both H5N1/04 influenza viruses significantly up-regulated IP-10, RANTES and IL-6 (p < 0.001) and interferon beta (p < 0.01) at 6 hours post-infection (Bonferroni multiple comparison test). Both H5N1/04 viruses significantly up-regulated MCP-1 and IL-8 to levels higher than H1N1 and H5N1/97 infected cells (p < 0.05, Bonferroni multiple comparison test). The mRNA concentrations of cytokine and chemokine mRNA were normalized to those β-actin mRNA in the corresponding samples. Means and standard deviation of duplicate cultures and assays are shown.
Figure 8
IP-10, Interleukin-6 and RANTES production by primary human bronchial and alveolar epithelial cells infected with A/Hong Kong/483/97 (H5N1/97), A/Vietnam/1194/04, A/Vietnam/3046/04 (both H5N1/04) and A/Hong Kong 54/98 (H1N1) influenza viruses or in mock infected cells. Culture supernatants from influenza virus-infected human respiratory epithelial cells collected at 3 h, 6 h and 24 h after infection with H5N1 and H1N1 viruses were tested by ELISA for IP-10 (Figure 8), Interleukin-6 (Figure 9) and RANTES (Figure 10). The IP-10, Interleukin-6 and RANTES mRNA levels were assayed at 3 h and 6 h post infection (data not shown) with results comparable with that shown in figure 5 and 7. The results from bronchial epithelial cells represent the means and standard deviations of three independent experiments (from the same donor). The means and standard deviations of the results from alveolar epithelial cells are based on experiments from six separate donors. * indicates p < 0.01 compared with mock and ** indicates p < 0.05 compared with H5N1/97 and H1N1 infected cells using the Bonferroni multiple comparison test.
Figure 9
IP-10, Interleukin-6 and RANTES production by primary human bronchial and alveolar epithelial cells infected with A/Hong Kong/483/97 (H5N1/97), A/Vietnam/1194/04, A/Vietnam/3046/04 (both H5N1/04) and A/Hong Kong 54/98 (H1N1) influenza viruses or in mock infected cells. Culture supernatants from influenza virus-infected human respiratory epithelial cells collected at 3 h, 6 h and 24 h after infection with H5N1 and H1N1 viruses were tested by ELISA for IP-10 (Figure 8), Interleukin-6 (Figure 9) and RANTES (Figure 10). The IP-10, Interleukin-6 and RANTES mRNA levels were assayed at 3 h and 6 h post infection (data not shown) with results comparable with that shown in figure 5 and 7. The results from bronchial epithelial cells represent the means and standard deviations of three independent experiments (from the same donor). The means and standard deviations of the results from alveolar epithelial cells are based on experiments from six separate donors. * indicates p < 0.01 compared with mock and ** indicates p < 0.05 compared with H5N1/97 and H1N1 infected cells using the Bonferroni multiple comparison test.
Figure 10
IP-10, Interleukin-6 and RANTES production by primary human bronchial and alveolar epithelial cells infected with A/Hong Kong/483/97 (H5N1/97), A/Vietnam/1194/04, A/Vietnam/3046/04 (both H5N1/04) and A/Hong Kong 54/98 (H1N1) influenza viruses or in mock infected cells. Culture supernatants from influenza virus-infected human respiratory epithelial cells collected at 3 h, 6 h and 24 h after infection with H5N1 and H1N1 viruses were tested by ELISA for IP-10 (Figure 8), Interleukin-6 (Figure 9) and RANTES (Figure 10). The IP-10, Interleukin-6 and RANTES mRNA levels were assayed at 3 h and 6 h post infection (data not shown) with results comparable with that shown in figure 5 and 7. The results from bronchial epithelial cells represent the means and standard deviations of three independent experiments (from the same donor). The means and standard deviations of the results from alveolar epithelial cells are based on experiments from six separate donors. * indicates p < 0.01 compared with mock and ** indicates p < 0.05 compared with H5N1/97 and H1N1 infected cells using the Bonferroni multiple comparison test.
Similar articles
- Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells.
Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan YI, Nicholls JM, Peiris JS. Chan MC, et al. Respir Res. 2009 Oct 30;10(1):102. doi: 10.1186/1465-9921-10-102. Respir Res. 2009. PMID: 19874627 Free PMC article. - [Cytokine storm in avian influenza].
Us D. Us D. Mikrobiyol Bul. 2008 Apr;42(2):365-80. Mikrobiyol Bul. 2008. PMID: 18697437 Review. Turkish. - The hemagglutinin protein of influenza A/Vietnam/1203/2004 (H5N1) contributes to hyperinduction of proinflammatory cytokines in human epithelial cells.
Cheng X, Xu Q, Song E, Yang CF, Kemble G, Jin H. Cheng X, et al. Virology. 2010 Oct 10;406(1):28-36. doi: 10.1016/j.virol.2010.06.048. Epub 2010 Jul 27. Virology. 2010. PMID: 20667571 - Replication and pathogenesis of avian influenza A (H5N1) virus infection in polarised human bronchial and alveolar epithelium.
Chan MC, Chan RW, Tsao GS, Peiris JS. Chan MC, et al. Hong Kong Med J. 2013 Jun;19 Suppl 4:24-8. Hong Kong Med J. 2013. PMID: 23775183 - Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses.
Guarner J, Falcón-Escobedo R. Guarner J, et al. Arch Med Res. 2009 Nov;40(8):655-61. doi: 10.1016/j.arcmed.2009.10.001. Epub 2010 Jan 6. Arch Med Res. 2009. PMID: 20304252 Review.
Cited by
- Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells.
Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan YI, Nicholls JM, Peiris JS. Chan MC, et al. Respir Res. 2009 Oct 30;10(1):102. doi: 10.1186/1465-9921-10-102. Respir Res. 2009. PMID: 19874627 Free PMC article. - Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation.
Chan RW, Yuen KM, Yu WC, Ho CC, Nicholls JM, Peiris JS, Chan MC. Chan RW, et al. PLoS One. 2010 Jan 15;5(1):e8713. doi: 10.1371/journal.pone.0008713. PLoS One. 2010. PMID: 20090947 Free PMC article. - Epidemic influenza and vitamin D.
Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Cannell JJ, et al. Epidemiol Infect. 2006 Dec;134(6):1129-40. doi: 10.1017/S0950268806007175. Epub 2006 Sep 7. Epidemiol Infect. 2006. PMID: 16959053 Free PMC article. Review. - Combination chemotherapy for influenza.
Govorkova EA, Webster RG. Govorkova EA, et al. Viruses. 2010 Aug;2(8):1510-1529. doi: 10.3390/v2081510. Epub 2010 Jul 27. Viruses. 2010. PMID: 21994692 Free PMC article. - Conserved host response to highly pathogenic avian influenza virus infection in human cell culture, mouse and macaque model systems.
McDermott JE, Shankaran H, Eisfeld AJ, Belisle SE, Neuman G, Li C, McWeeney S, Sabourin C, Kawaoka Y, Katze MG, Waters KM. McDermott JE, et al. BMC Syst Biol. 2011 Nov 11;5:190. doi: 10.1186/1752-0509-5-190. BMC Syst Biol. 2011. PMID: 22074594 Free PMC article.
References
- Subbarao K, Klimov A, Katz J, Regenery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. - DOI - PubMed
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, Pham TS, Vo CD, Le TQ, Ngo TT, Dao BK, Le PP, Nguyen TT, Hoang TL, Cao VT, Le TG, Nguyen DT, Le HN, Nguyen KT, Le HS, Le VT, Christiane D, Tran TT, Menno de J, Schultsz C, Cheng P, Lim W, Horby P, Farrar J, World Health Organization International Avian Influenza Investigative Team Avian influenza A (H5N1) in 10 patients in Vietnam. N Eng J Med. 2004;350:1179–88. doi: 10.1056/NEJMoa040419. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical