Anaphase-promoting complex/cyclosome controls the stability of TPX2 during mitotic exit - PubMed (original) (raw)
Anaphase-promoting complex/cyclosome controls the stability of TPX2 during mitotic exit
Scott Stewart et al. Mol Cell Biol. 2005 Dec.
Abstract
TPX2, a microtubule-associated protein, is required downstream of Ran-GTP to induce spindle assembly. TPX2 activity appears to be tightly regulated during the cell cycle, and we report here one molecular mechanism for this regulation. We found that TPX2 protein levels are cell cycle regulated, peaking in mitosis and declining sharply during mitotic exit. TPX2 is degraded in mitotic extracts, as well as in HeLa cells exiting from mitosis. This instability depends, both in vitro and in vivo, on the anaphase-promoting complex/cyclosome (APC/C), a ubiquitin ligase that controls mitotic progression. In a reconstituted system, TPX2 is efficiently ubiquitinated by APC/C that has been activated by Cdh1. Two discrete elements in TPX2 are required for recognition by APC/C(Cdh1): a KEN box and a novel element in amino acids 1 to 86. Interestingly, the latter element, which has no known APC/C recognition motifs, is required for the ubiquitination of TPX2 by APC/C(Cdh1) in vitro and for its degradation in vivo. We conclude that APC/C(Cdh1) controls the stability of TPX2, thereby ensuring accurate regulation of the spindle assembly in the cell cycle.
Figures
FIG. 1.
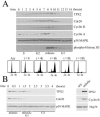
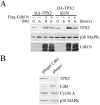
Levels of the TPX2 protein fluctuate in the cell cycle. (A) HeLa S3 cells were arrested at the G1/S boundary by a double-thymidine treatment. Cells were released into fresh medium and harvested every hour. Cell lysates were Western blotted with TPX2, Cdc20, cyclin B, cyclin A, p38 MAPK, and phosphohistone H3 antibodies. The p38 MAPK Western blot served as a loading control. Cell cycle stages were determined by propidium iodide staining and flow cytometry. (B) HeLa S3 cells were arrested at prometaphase through a thymidine-nocodazole treatment. Cells were released into fresh medium and harvested at the indicated times (left). Extracts from asynchronous cells (asy) and prometaphase-arrested cells (mitotic) were also directly compared (right). Cell lysates were Western blotted with TPX2, Cdc20, cyclin B, Hsp70, and p38 MAPK antibodies as indicated. Cell cycle stages were determined by propidium iodide staining and flow cytometry, as well as by DAPI staining and microscopy (data not shown).
FIG. 2.
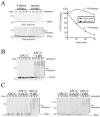
TPX2 is unstable in mitotic extracts and is ubiquitinated by APC/C in vitro. (A) HeLa S3 cells were synchronized in S phase with hydroxyurea or at late anaphase/telophase with a thymidine-nocodazole block, followed by a 1-hour release in fresh medium. The cells were lysed in a hypotonic buffer, and the stability of the 35S-labeled TPX2 was analyzed in the two extracts. TPX2 remaining at each time point was quantified, and half-lives (_t_1/2) were determined. (B) TPX2 is a substrate of APC/CCdh1 in vitro. APC/C was immunopurified from Xenopus interphase extracts and activated with recombinant Cdh1. APC/C-dependent ubiquitination of 35S-labeled TPX2 was analyzed in the presence of E1, E2, ubiquitin (Ub), ubiquitin-aldehyde, and an energy mix. (C) Comparison of APC/CCdh1 and APC/CCdc20 activities toward TPX2. APC/C was immunopurified from Xenopus interphase extracts and activated with recombinant Cdh1 or Cdc20. APC/C-dependent ubiquitination of 35S-labeled TPX2 or Plk-1 was analyzed in the presence of E1, E2, ubiquitin, ubiquitin-aldehyde, and an energy mix. Plk-1, a physiological target of APC/C Cdh1, was included as a substrate under identical experimental conditions for comparative purposes.
FIG. 3.
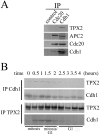
Cell-cycle-dependent association of TPX2 and Cdh1 (A) Cdh1, not Cdc20, associates with TPX2. Extracts were prepared from cells after a 2-hour release from a thymidine-nocodazole block and subsequently immunoprecipitated with Cdc20, Cdh1 antibodies, or control rabbit IgG. Immune complexes were then analyzed by Western blotting with the indicated antibodies. APC2 is a subunit of APC/C. (B) Cdh1 and TPX2 associate in late mitosis. HeLa S3 cells were synchronized by a thymidine-nocodazole block and released for the times indicated. Cdh1 and TPX2 were immunoprecipitated (IP) with respective antibodies, and the immune complexes were analyzed by Western blotting with the indicated antibodies. Cell cycle stages were determined by propidium iodide staining and flow cytometry. Total levels of TPX2 in these extracts are shown in Fig. 1B.
FIG. 4.
Degradation of TPX2 in mitotic extracts is dependent on APC/C. (A) Mitotic extracts were prepared from cells harvested 1 hour post-thymidine-nocodazole release. Extracts were first incubated with control or α-Cdc27 antibodies or with 100 μg/ml recombinant Emi1 and then assayed for degradation of TPX2 (top). The extent of degradation was quantified as in Fig. 2 (bottom). (B) Western blot demonstrating the extent and specificity of depletion of Cdc27. “Supernatant” contains 5 μl of depleted or mock-depleted extracts, and “beads” contain immune complexes from 5 μl of extracts. The Cdc27 Western blot shows efficient removal of Cdc27 from extracts by the Cdc27 antibody. The p38 MAPK Western blot illustrates that p38 MAPK was not depleted by either antibody.
FIG. 5.
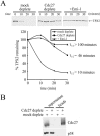
The stability of TPX2 is under the control of the APC/C pathway in vivo. (A) Stabilization of TPX2 and TPX2 KEN by dominant-negative Cdh1. HeLa cells were transfected with expression vectors for HA-TPX2 or HA-TPX2 KEN in the presence or absence of Flag-Cdh1N. Cells were synchronized by a thymidine-nocodazole block and released for the times indicated. Total cell lysates were analyzed by Western blotting. HA-tagged TPX2 proteins were detected with an HA antibody, and Cdh1N was detected with a Flag antibody. The p38 MAPK Western blot served as a loading control. At T = 0, all cells were arrested at prometaphase, and at T = 6 h, all cells had progressed into G1 with similar kinetics (data not shown). (B) Knockdown of Cdh1 stabilizes TPX2. Expression of Cdh1 was blocked by transfection of pSuper-Cdh1b into HeLa cells. Levels of TPX2, Cdh1, cyclin A, and p38 MAPK were analyzed 60 h posttransfection by Western blotting.
FIG. 6.
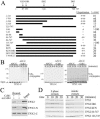
Recognition of TPX2 by APC/CCdh1. (A) Schematic representation of wild-type TPX2 and summary of ubiquitination assays and half-life (t1/2) measurement for various TPX2 mutants. Reactions were performed as described in the legend to Fig. 2. The extent of ubiquitination was semiquantified by assigning a value of either “+++” (full activity), “++” (moderate activity), “+” (detectable activity), or “−” (no detectable activity), examples of which are provided in panel B. 1-704 K12 is a TPX2 mutant lacking the KEN box and all three D boxes. Half-lives were determined as in Fig. 2 and are indicated in minutes. nd, not determined. (B) The amino-terminal region of TPX2 is recognized by APC/CCdh1. TPX2-N (amino acids 1 to 324), TPX2-C (amino acids 325 to 747), and TPX2 1-86 were analyzed in the reconstituted ubiquitination reaction described in the legend to Fig. 2B. (C) Binding of Cdh1 to TPX2 and TPX2-N. TPX2, TPX2-N, and TPX2-C were in vitro translated and then incubated with or without recombinant Cdh1. The Cdh1 complexes were then immunoprecipitated by anti-Cdh1 antibodies, washed, and analyzed by SDS-PAGE. The input lane contained 2% of the in vitro-translated sample used in each binding reaction. TPX2-C failed to specifically associate with Cdh1 above nonspecific background. (D) Stabilities of TPX2 mutants in mitotic extracts. The stabilities of the deletion and point mutants of TPX2 were analyzed in mitotic extracts as described in the legend to Fig. 2A. Ub, ubiquitin.
FIG. 7.
Stability of wild type and mutants of TPX2 in mitosis in vivo. HeLa cells were transiently transfected with the indicated HA-tagged TPX2 expression vector (0.2 μg/10-cm dish). Transfected cells were arrested by a thymidine-nocodazole block and then released into fresh medium containing 10 μg/ml cycloheximide. Cells were harvested at the times indicated, and the half-lives of expressed proteins were determined by Western blot analysis. The HA-tagged TPX2 proteins were detected with HA antibody, and GFP-TPX2 was detected with TPX2 antibody. The p38 MAPK Western blot served as a loading control. Cell cycle stages were determined by DAPI staining and microscopy. Low expression levels of TPX2 and TPX2 mutants in this experiment did not alter mitotic exit and progression into G1.
FIG. 8.
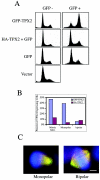
Expression of GFP-TPX2 resulted in accumulation of prometaphase cells with abnormal spindles. (A) HeLa cells were transfected with GFP-TPX2 fusion (first row), with HA-TPX2 plus GFP (nine parts of HA-GFP with one part of GFP; second row), with GFP alone (third row), or with HA control vector (fourth row). The cells were fixed at 30 h posttransfection, stained with propidium iodide, and subjected to flow cytometry. Fluorescence-activated cell sorter profiles of the GFP-positive (right) and GFP-negative (left) cells are shown here. (B) HeLa cells were transfected with GFP-TPX2 or HA-TPX2, and the cells were fixed at 30 h posttransfection and stained with either HA or GFP antibodies, together with DAPI and an anti-β-tubulin antibody. The mitotic index and percentages of monopolar and bipolar cells were quantified in the transfected cells. (C) HeLa cells were transfected with GFP-TPX2, and the cells were fixed at 30 h posttransfection and stained with DAPI and β-tubulin antibodies. GFP-TPX2 is in green, DAPI is in blue, and β-tubulin is in red. Bar = 5 μm.
Similar articles
- Regulated degradation of spindle assembly factors by the anaphase-promoting complex.
Song L, Rape M. Song L, et al. Mol Cell. 2010 May 14;38(3):369-82. doi: 10.1016/j.molcel.2010.02.038. Mol Cell. 2010. PMID: 20471943 Free PMC article. - Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1.
Stewart S, Fang G. Stewart S, et al. Cancer Res. 2005 Oct 1;65(19):8730-5. doi: 10.1158/0008-5472.CAN-05-1500. Cancer Res. 2005. PMID: 16204042 - KEN-box-dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase-promoting complex/cyclosome.
Qi W, Yu H. Qi W, et al. J Biol Chem. 2007 Feb 9;282(6):3672-9. doi: 10.1074/jbc.M609376200. Epub 2006 Dec 11. J Biol Chem. 2007. PMID: 17158872 - Non-mitotic functions of the Anaphase-Promoting Complex.
Eguren M, Manchado E, Malumbres M. Eguren M, et al. Semin Cell Dev Biol. 2011 Aug;22(6):572-8. doi: 10.1016/j.semcdb.2011.03.010. Epub 2011 Mar 23. Semin Cell Dev Biol. 2011. PMID: 21439391 Review. - Mitotic regulation of the anaphase-promoting complex.
Baker DJ, Dawlaty MM, Galardy P, van Deursen JM. Baker DJ, et al. Cell Mol Life Sci. 2007 Mar;64(5):589-600. doi: 10.1007/s00018-007-6443-1. Cell Mol Life Sci. 2007. PMID: 17334950 Free PMC article. Review.
Cited by
- Insights into APC/C: from cellular function to diseases and therapeutics.
Zhou Z, He M, Shah AA, Wan Y. Zhou Z, et al. Cell Div. 2016 Jul 13;11:9. doi: 10.1186/s13008-016-0021-6. eCollection 2016. Cell Div. 2016. PMID: 27418942 Free PMC article. Review. - The function of APC/CCdh1 in cell cycle and beyond.
Li M, Zhang P. Li M, et al. Cell Div. 2009 Jan 19;4:2. doi: 10.1186/1747-1028-4-2. Cell Div. 2009. PMID: 19152694 Free PMC article. - TPX2 is required for postmitotic nuclear assembly in cell-free Xenopus laevis egg extracts.
O'Brien LL, Wiese C. O'Brien LL, et al. J Cell Biol. 2006 Jun 5;173(5):685-94. doi: 10.1083/jcb.200512107. Epub 2006 May 30. J Cell Biol. 2006. PMID: 16735579 Free PMC article. - TPX2 is a novel prognostic marker for the growth and metastasis of colon cancer.
Wei P, Zhang N, Xu Y, Li X, Shi D, Wang Y, Li D, Cai S. Wei P, et al. J Transl Med. 2013 Dec 17;11:313. doi: 10.1186/1479-5876-11-313. J Transl Med. 2013. PMID: 24341487 Free PMC article. - Regulated degradation of spindle assembly factors by the anaphase-promoting complex.
Song L, Rape M. Song L, et al. Mol Cell. 2010 May 14;38(3):369-82. doi: 10.1016/j.molcel.2010.02.038. Mol Cell. 2010. PMID: 20471943 Free PMC article.
References
- Bayliss, R., T. Sardon, I. Vernos, and E. Conti. 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12:851-862. - PubMed
- Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. - PubMed
- Carazo-Salas, R. E., G. Guarguaglini, O. J. Gruss, A. Segref, E. Karsenti, and I. W. Mattaj. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400:178-181. - PubMed
- Eyers, P. A., E. Erikson, L. G. Chen, and J. L. Maller. 2003. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 13:691-697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous