Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing - PubMed (original) (raw)
Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing
Li-Xin Wang et al. Cancer Res. 2005.
Abstract
Active-specific immunotherapy with dendritic cells loaded with peptide derived from the melanoma antigen, gp100, failed to mediate regression of established B16F10 melanoma in normal mice. Dendritic cell vaccination induced activation and subsequent deletion of adoptively transferred naive CD8+ T-cell receptor transgenic (pmel-1) T cells specific for gp100 in normal mice. In lymphodepleted mice, dendritic cell vaccination produced greater T-cell expansion, long-term persistence of memory T cells, and tumor regression. Most tumors that persisted in the presence of functional memory T cells had either lost or exhibited reduced expression of MHC class I or gp100 proteins. In contrast to other naive T cells, pmel-1 T cells adoptively transferred to lymphodepleted mice exhibited faster proliferation and a more differentiated phenotype after exposure to peptide-pulsed dendritic cells. Proliferation and persistence of pmel-1 T cells was highly dependent on interleukin-7 (IL-7) in irradiated mice, and IL-15 when IL-7 was neutralized, two critical homeostatic cytokines produced in response to the irradiation-induced lymphodepletion.
Figures
Figure 1
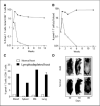
Expansion and persistence of pmel-1 transgenic T cells in lymphodepleted mice after vaccination. Sublethal irradiation of C57BL/6 (CD45.2+) mice was followed by adoptive transfer of 106 pmel-1 GFP double transgenic spleen cells together with 10 × 106 naive C57BL/6 (CD45.1+) spleen cells and vaccination with 1 × 106 hgp-9 peptide-pulsed dendritic cells. Blood was collected at different weeks after vaccination, and the percentage (A) and absolute number (B) of pmel-1 T cells (CD8+GFP+CD45.1−) in pooled blood samples (n = 5) were determined by flow cytometry analysis. The percentage of pmel-1 transgenic T cells among CD8+ T cells in the spleen, bone marrow (BM), and lung were compared with that of blood (C); rapid development of depigmentation was observed in irradiated mice (D). Representative of at least three independent experiments.
Figure 2
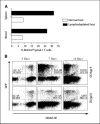
Kinetics of antigen-driven and homeostatic proliferation–driven proliferation of T cells in lymphodepleted mice. A, at the peak of their response, a higher percentage of pmel-1 T cells incorporated BrdUrd in lymphodepleted mice than in normal mice after vaccination. BrdUrd was administered to normal and lymphodepleted mice 7 days after adoptive transfer and vaccination. The percentage of pmel-1 transgenic T cells in both blood and spleen that had incorporated BrdUrd was determined by intracellular staining with APC-conjugated anti-BrdUrd antibody (BD PharMingen, San Diego, CA), and flow cytometry analysis was done according to the manufacturer's protocol. B, pmel-1 transgenic T cells from irradiated mice lost completely DDAO-SE labeling 1 week after vaccination with dendritic cells (DC) loaded with cognate peptide hgp-9. It took 2 weeks to lose DDAO-SE labeling when mice were vaccinated with dendritic cells loaded with control peptide gp33. Congenic nontransgenic T cells also needed at least 2 weeks to dilute all of their DDAO-SE labeling when transferred into irradiated vaccinated with either hgp-9 or gp33 peptide-loaded dendritic cells. Before adoptive transfer, pmel-1 and congenic spleen cells were labeled with 10 μmol/L DDAO-SE and adoptively transferred into mice shortly after irradiation. One group of mice was vaccinated with hgp-9-loaded dendritic cells, whereas the other group of mice was vaccinated with gp33-loaded dendritic cells. Mice were sacrificed at indicated time points, and spleen cells were analyzed by flow cytometry.
Figure 3
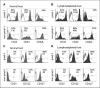
Phenotype of adoptively transferred T cells obtained from vaccinated normal and lymphodepleted mice. Seven days after adoptive transfer and vaccination, T cells were obtained from the blood of normal (A and C) and lymphodepleted (B and D) mice. pmel-1 transgenic T cells (CD8+GFP+CD45.1−) and congenic nontransgenic T cells (CD8+GFP−CD45.1+) were gated on GFP and CD45 expression, and then the expression of memory markers (A and B: CD43, CD44, and CD62L) and cytokine receptors (C and D: CD25, CD122, and CD127) was determined using PE-conjugated antibodies. Open histograms, staining with isotype controls; filled histograms, staining with specific antibodies. The percentage and number indicated in each histogram represent the percentage of positive cells and the mean florescence intensity of staining, respectively.
Figure 4
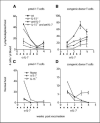
IL-7-dependent expansion and persistence of pmel-1 T cells in lymphodepleted mice. Irradiated (A-B) and nonirradiated (C and D) WT C57BL/6 and IL-15−/− mice were vaccinated with hgp-9-loaded dendritic cells after the adoptive transfer of pmel-1 T cells. Some mice also received 1 mg anti-mouse IL-7 monoclonal antibody (M2) i.p. twice weekly for 3 weeks. The number of circulating pmel-1 transgenic T cells (CD8+GFP−CD45.1−) and CD8+ congenic nontransgenic T cells (CD8+GFP−CD45.1+) was determined by flow cytometry analysis at the indicated time points. Bars, SD from the number obtained from three to five mice per group. One of two experiments.
Figure 5
Functional characterization of pmel-1 T cells. A, the majority of pmel-1 transgenic T cells at the peak of response from both normal and lymphodepleted mice produced IFN-γ upon stimulation with hgp-9 peptide ex vivo. A higher percentage of nontransgenic CD8+ T cells from lymphodepleted mice compared to normal mice produced IFN-γ. One of three experiments. The percentage was derived from pooled blood from three to five mice. B, persistence of pmel-1 and non-pmel-1 tetramer-positive T cells in vaccinated lymphodepleted mice. Blood was collected from normal and lymphodepleted mice 70 days after vaccination and stained with hgp-9/Db MHC tetramers. Data were for lymphodepleted mice only, because there were no tetramer-positive cells in normal mice 70 days after vaccination, and no secondary response when normal mice were boostered with dendritic cell/hgp-9 again. The numbers indicate the percentage of tetramer-positive cells from peml-1 transgenic T cells or nontransgenic CD8+ T cells from pooled blood of three to five mice. C, in vivo killing of hgp-9-coated spleen cells. Naive spleen cells coated with 10 μg/mL of hgp-9 peptide and labeled with 10 μmol/L CFSE were mixed with naive spleen cells pulsed with the gp33 peptide of LCMV and labeled with 2 μmol/L CFSE before injection into normal or lymphodepleted mice 55 days after adoptive transfer and vaccination. The numbers represent the percentage of killing of peptide-coated spleen cells in one of three mice in each experiment. Representative of one of three independent experiments. D, secondary responses of pmel-1 T cells in vaccinated lymphodepleted mice. Two weeks after the primary vaccination, mice were subjected to a secondary booster vaccine. The numbers of pmel-1 transgenic T cells in the blood were determined at different time points before and after the booster vaccine. Representative of one of three independent experiments.
Figure 6
Tumor regression and escape in lymphodepleted mice after adoptive transfer and vaccination. A and B, naive C57BL/6 mice were injected with 2 × 105 B16-F10 tumor cells s.c. at day 0. Five days later, half the mice were irradiated. Both normal and irradiated mice were vaccinated with 1 × 106 dendritic cells (DC) loaded with hgp-9 peptide s.c. at day 6 after adoptive transfer of pmel-1 transgenic and nontransgenic congenic naive T cells. Booster vaccines were given 20 days after tumor injection. Control mice received T-cell transfer but no vaccine. Tumor was measured thrice each week. Mice were sacrificed when the longest tumor diameter was >15 mm. A, tumor area. B, survival. Representative of one of three independent experiments. C-D, F10 escape variants were resistant to vaccines, indicating an immunoediting process that actively sculpting the antigen profiles of tumor cells. Dendritic cells loaded with either hgp-9 or gp33 failed to affect the growth (C) or the survival (D) of F10 variant #439 (lost MHC) and #442 (lost go100). Inset, table list of the median survival in days calculated by Kaplan-Meier survival curve analysis. Western blot analysis with anti-tyrosinase antibody showed that each of these tumor cells (#438, #439, and #442) continued to express tyrosinase (E). Human melanoma cell line FEMX and parental F10 were included as the positive control.
Similar articles
- Toll-like receptors in tumor immunotherapy.
Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer DC, Yu Z, Antony PA, Gattinoni L, Rosenberg SA, Restifo NP. Paulos CM, et al. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5280-9. doi: 10.1158/1078-0432.CCR-07-1378. Clin Cancer Res. 2007. PMID: 17875756 Free PMC article. Review. - Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice.
Bullock TN, Mullins DW, Colella TA, Engelhard VH. Bullock TN, et al. J Immunol. 2001 Nov 15;167(10):5824-31. doi: 10.4049/jimmunol.167.10.5824. J Immunol. 2001. PMID: 11698456 - Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo.
Lou Y, Wang G, Lizée G, Kim GJ, Finkelstein SE, Feng C, Restifo NP, Hwu P. Lou Y, et al. Cancer Res. 2004 Sep 15;64(18):6783-90. doi: 10.1158/0008-5472.CAN-04-1621. Cancer Res. 2004. PMID: 15374997 Free PMC article. - Effective induction of therapeutic antitumor immunity by dendritic cells coexpressing interleukin-18 and tumor antigen.
Xia D, Zheng S, Zhang W, He L, Wang Q, Pan J, Zhang L, Wang J, Cao X. Xia D, et al. J Mol Med (Berl). 2003 Sep;81(9):585-96. doi: 10.1007/s00109-003-0472-5. Epub 2003 Aug 21. J Mol Med (Berl). 2003. PMID: 12937899 - IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells.
Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP. Klebanoff CA, et al. Proc Natl Acad Sci U S A. 2004 Feb 17;101(7):1969-74. doi: 10.1073/pnas.0307298101. Epub 2004 Feb 4. Proc Natl Acad Sci U S A. 2004. PMID: 14762166 Free PMC article.
Cited by
- Improving TCR Gene Therapy for Treatment of Haematological Malignancies.
Nicholson E, Ghorashian S, Stauss H. Nicholson E, et al. Adv Hematol. 2012;2012:404081. doi: 10.1155/2012/404081. Epub 2012 Jan 26. Adv Hematol. 2012. PMID: 22319532 Free PMC article. - Toll-like receptors in tumor immunotherapy.
Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer DC, Yu Z, Antony PA, Gattinoni L, Rosenberg SA, Restifo NP. Paulos CM, et al. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5280-9. doi: 10.1158/1078-0432.CCR-07-1378. Clin Cancer Res. 2007. PMID: 17875756 Free PMC article. Review. - The allure and peril of hematopoietic stem cell transplantation: overcoming immune challenges to improve success.
Newman RG, Ross DB, Barreras H, Herretes S, Podack ER, Komanduri KV, Perez VL, Levy RB. Newman RG, et al. Immunol Res. 2013 Dec;57(1-3):125-39. doi: 10.1007/s12026-013-8450-7. Immunol Res. 2013. PMID: 24272856 Free PMC article. Review. - CD8+ T-cell memory in tumor immunology and immunotherapy.
Klebanoff CA, Gattinoni L, Restifo NP. Klebanoff CA, et al. Immunol Rev. 2006 Jun;211:214-24. doi: 10.1111/j.0105-2896.2006.00391.x. Immunol Rev. 2006. PMID: 16824130 Free PMC article. Review. - Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors.
Mescher MF, Popescu FE, Gerner M, Hammerbeck CD, Curtsinger JM. Mescher MF, et al. Semin Cancer Biol. 2007 Aug;17(4):299-308. doi: 10.1016/j.semcancer.2007.06.008. Epub 2007 Jun 23. Semin Cancer Biol. 2007. PMID: 17656106 Free PMC article. Review.
References
- Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–16. - PubMed
- Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA107243/CA/NCI NIH HHS/United States
- Z01 BC010763-01/Intramural NIH HHS/United States
- Z99 CA999999/Intramural NIH HHS/United States
- R01-CA107243/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials