Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy - PubMed (original) (raw)
Comparative Study
Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy
Clifford W Shults et al. J Neurosci. 2005.
Abstract
Multiple system atrophy (MSA) is a progressive, neurodegenerative disease characterized by parkinsonism, ataxia, autonomic dysfunction, and accumulation of alpha-synuclein (alpha-syn) in oligodendrocytes. To better understand the mechanisms of neurodegeneration and the role of alpha-syn accumulation in oligodendrocytes in the pathogenesis of MSA, we generated transgenic mouse lines expressing human (h) alpha-syn under the control of the murine myelin basic protein promoter. Transgenic mice expressing high levels of halpha-syn displayed severe neurological alterations and died prematurely at 6 months of age. Furthermore, mice developed progressive accumulation of halpha-syn-immunoreactive inclusions in oligodendrocytes along the axonal tracts in the brainstem, basal ganglia, cerebellum, corpus callosum, and neocortex. The inclusions also reacted with antibodies against phospho-serine (129) halpha-syn and ubiquitin, and halpha-syn was found in the detergent-insoluble fraction. In high-expresser lines, the white matter tracts displayed intense astrogliosis, myelin pallor, and decreased neurofilament immunostaining. Accumulation of halpha-syn in oligodendrocytes also leads to prominent neurodegenerative changes in the neocortex with decreased dendritic density and to loss of dopaminergic fibers in the basal ganglia. The oligodendrocytic inclusions were composed of fibrils and accompanied by mitochondrial alterations and disruption of the myelin lamina in the axons. Together, these studies support the contention that accumulation of alpha-syn in oligodendrocytes promotes neurodegeneration and recapitulates several of the key functional and neuropathological features of MSA.
Figures
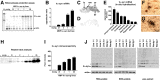
Figure 1.
Characterization of hα-syn expression under the MBP promoter in tg mice. A, RNase protection assay. Representative autoradiograph of hα-syn mRNA showing that levels were highest in line 29 mice, followed by lines 1, 2, and 31. The left lane shows signals of undigested (U) radiolabeled riboprobes (identified on left); the other lanes contain the same riboprobes plus brain RNA samples digested with RNases. Protected mRNAs are indicated on the right. B, Semiquantitative analysis of levels of hα-syn mRNA expression in tg mice; results are expressed as a ratio of hα-syn to actin. Bars are mean ± SEM. C, In situ hybridization with an antisense probe shows intense hybridization in the white matter tracts (wm), cerebellum (ce), and brainstem (bs), and to a lesser extent in the frontal cortex (fc) of an MBP hα-syn tg mouse from line 29. D, No hybridization is observed with a sense probe in an MBP hα-syn tg mouse from line 29. E, Image analysis of the levels of hybridization in various brain regions in an MBP hα-syn tg mouse from line 29. F, G, Higher-magnification view of the hybridization signal in glial cells in the corpus callosum, in which hα-syn mRNA (black grains) was colocalized with the oligodendroglial marker GC (brown; F), and hα-syn mRNA (black grains) was colocalized with hα-syn immunoreactivity (brown) in oligodendroglial cells (G). Scale bar: (in G)20 μm. H, Western blot analysis with the hα-syn antibody that identifies monomeric hα-syn as a single band at ∼14 kDa. I, Semiquantitative analysis of levels of hα-syn immunoreactivity in tg lines; results are expressed as integrated pixel intensity. J, Immunoblot analysis of the detergent-soluble and -insoluble distribution of hα-syn. Error bars are mean ± SEM.
Figure 2.
Patterns of hα-syn immunoreactivity in the brains of MBP hα-syn tg mice. All panels are images of the specified brain regions of vibratome sections of 4-month-old tg mice from line 31 (A-E), line 2 (F-J), line 1 (K-O), or line 29 (P-T) immunostained with an antibody against hα-syn, developed with DAB, and analyzed by bright-field microscopy. A, B, F, G, K, L, P, Q, Oligodendroglial cells in the neocortex of tg mice from line 31 (A, B), line 2 (F, G), and line 1 (K, L) at low (200×; A, F, K) and high (400×; B, G, L) magnification (mag) show abundant hα-syn immunoreactivity. C-E, H-J, M-O, The basal ganglia (C, H, M), corpus callosum (D, I, N), and cerebellum (E, J, O) of line 31, line 2, and line 1 tg mice also display abundant oligodendroglial cells with hα-syn immunoreactivity. P, Q, Oligodendroglial cells in the neocortex of tg mice of the higher-expresser line 29 at low (P) and high (Q) magnification show a higher density of hα-syn-immunoreactive inclusions and vacuolization and expansion of the cytoplasm (arrow). R-T, The basal ganglia (R), corpus callosum (S), and cerebellum (T) of line 29 tg mice also show a higher density of hα-syn-immunoreactive oligodendroglial cells. Scale bars: (in T) A, C-E, F, H-J, K, M-O, P, R-T, 100 μm; (in Q) B, G, L, Q, 10 μm.
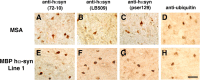
Figure 3.
Comparison of the glial cell inclusions between MSA and MBP hα-syn tg animals. Images are from the white matter tracts in the basal ganglia of a human case with typical MSA and MBP hα-syn tg mice from line 1 (4 months of age). A, Conical and ovoid GCIs in an MSA case were positively immunostained with a polyclonal antibody against hα-syn (72-10). B, GCIs immunostained with a monoclonal antibody against hα-syn (LB509). C, GCIs immunostained with a monoclonal antibody against phospho-serine129 hα-syn (pser129). D, GCIs immunostained with an antibody against ubiquitin. E, Conical and ovoid glial inclusions in an MBP hα-syn tg mouse were positively immunostained with a polyclonal antibody against hα-syn (72-10). F, Glial inclusions immunostained with a monoclonal antibody against hα-syn (LB509). G, Glial inclusions immunostained with a monoclonal antibody against phospho-serine129 hα-syn (pser129). H, Glial inclusions in the tg mice were mildly immunostained with an antibody against ubiquitin. Scale bar, 20 μm.
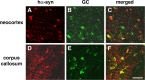
Figure 4.
Colocalization of hα-syn with oligodendrocytic markers in the brains of MBP hα-syn tg mice. All images are from brain sections of 4-month-old tg mice from line 29 immunostained with antibodies against hα-syn and the oligodendroglial cell marker GC and imaged with the LSCM. A-C are images from the neocortex, and D-F are from the corpus callosum. In both the neocortex and corpus callosum, hα-syn-immunoreactive cells (red) also display galactocerebroside immunolabeling (green), as indicated by arrows, and colocalization is indicated by the yellow in the merged images. Scale bar, 40 μm.
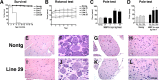
Figure 5.
Characterization of survival and neurological deficits in MBP hα-syn tg mice. A, Survival curves for the MBP hα-syn tg mice showing that, although mice from the lower-expresser lines (1, 2, and 31) were viable for long periods of time, mice from high-expresser line 29 died prematurely by 6 months of age. B, Motor assessment in the rotarod showed that compared with nontg mice (n = 6), tg mice from line 31 (n = 6) had a mild impairment of motor function, whereas tg mice from lines 2 (n = 6), 1 (n = 6), and 29 (n = 6) had more significant motor deficits in this test. Mice were tested at 4 months of age. C, Motor assessment in the pole test showed that compared with nontg mice (n = 6), tg mice from lines 31 (n = 6) and 2 (n = 6) demonstrated motor abilities comparable with control mice, whereas tg mice from higher-expresser lines 1 (n = 6) and 29 (n = 6) displayed significant motor impairment. Mice were tested at 4 months of age. D, Motor assessment in the pole test showed that, at 3 months of age, only mild deficits were observed; however, at 6 months of age, these deficits were accentuated and remained similar at 12 months of age. Error bars are mean ± SEM. *Significant difference compared with nontg controls (p < 0.05; one-way ANOVA with post hoc Dunnett's). E-L, For histological analysis, paraffin sections from 4-month-old mice were stained with hematoxylin/eosin and imaged by bright-field microscopy. E-H, Histological analysis of the hindlimb muscle (E), dorsal root ganglion (F), spinal nerve roots (G), and motor neurons (H) in the thoracic segments of the spinal cord in nontg mice. I-L, No significant alterations were observed in muscle (I), dorsal root ganglion (J), spinal nerve roots (K), and motor neurons (L) in the thoracic segments of the spinal cord in MBP hα-syn tg mice. Scale bar, 20 μm.
Figure 6.
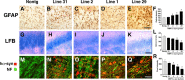
Neuropathological alterations in the corpus callosum of MBP hα-syn tg mice. All panels are from vibratome sections from the brains of 4-month-old mice immunostained with an antibody against GFAP (A-F) or the histochemical stain LFB (G-L) and imaged by bright-field microscopy or with antibodies against hα-syn and NF and imaged with the LSCM (M-R). A-E, Immunocytochemical analysis with an antibody against GFAP shows that compared with a nontg control (A), tg mice from all four lines (B-E) displayed astrogliosis in the corpus callosum. F, Semiquantitative analysis of levels of astrogliosis, as measured by GFAP immunoreactivity. Lines 2, 1, and 29 display more intense astrogliosis compared with nontg controls and lower-expresser line 31. G-K, Staining with LFB demonstrates that compared with a nontg control (G), in MBP hα-syn tg mice (H-K), myelin staining of this region was reduced. L, Semiquantitative analysis of levels of myelin staining, as measured by LFB reactivity. High-expresser lines 1 and 29 show decreased myelin staining compared with nontg controls and low-expresser lines 31 and 2. M-Q, Double immunocytochemical analysis shows that compared with a nontg control (M), hα-syn immunoreactivity (red) was accompanied by extensive axonal (NF; green) alterations, including decreased neurite density and formation of irregular and enlarged axons (arrows) (N-Q). R, Semiquantitative analysis of levels of axonal integrity, as measured by NF immunoreactivity. Lines 2, 1, and 29 display more significant decreases in levels of NF immunoreactivity compared with nontg controls and low-expresser line 31. Scale bars: (in E) A-E, 50 μm; (in K) G-K, 100 μm; (in Q) M-Q, 20 μm. Error bars are mean ± SEM. The asterisks indicate significant difference compared with nontg controls (p < 0.05; one-way ANOVA with post hoc Dunnett's).
Figure 7.
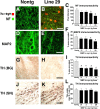
Patterns of neurodegeneration in the neocortex and dopaminergic system of MBP hα-syn tg mice. All panels are from the neocortex of 4-month-old mice. Sections were labeled with antibodies against hα-syn and NF or MAP2 and imaged with the LSCM or with an antibody against TH and imaged by bright-field microscopy. A, B, NF immunoreactivity demonstrates that compared with a nontg control (A), hα-syn immunoreactivity in oligodendrocytes (arrows) in the neocortex of tg mice (B) is accompanied by axonal atrophy and a decrease in the density of neuritic processes, particularly in the high-expresser line 29 (B). C, Levels of axonal integrity, as measured by NF immunoreactivity. Lines 2, 1, and 29 display more significant decreases in levels of NF immunoreactivity compared with nontg controls and low-expresser line 31. D-E, MAP2 immunoreactivity shows that compared with a nontg control (D), MBP hα-syn tg mice (E) had extensive damage to dendrites and decreased density of dendritic structures in the neocortex, particularly in the higher-expresser line 29 (E). F, Confocal analysis of the percentage of the area of the neuropil occupied by MAP2-immunoreactivite dendrites. High-expresser lines 1 and 29 demonstrate a significant decrease in MAP2 immunoreactivity compared with nontg controls and lower-expresser lines 31 and 2. G, H, Compared with a nontg control (G), MBP hα-syn tg mice (H) showed decreased TH-immunoreactive fibers in the basal ganglia (BG). I, Computer-aided image analysis of percentage of area occupied by TH-immunolabeled fibers. All tg lines demonstrate decreased levels of TH-immunoreactive fibers. J, K, TH immunoreactivity in neurons in the SN in nontg (J) and MBP hα-syn tg (K) mice. L, Image analysis of the numbers of TH-positive neurons shows no differences among the groups. sq, Square. Scale bars: (in B, E) A-E, 20 μm; (in H) G, H, 30 μm; (in K) J, K, 40 μm. Error bars are mean ± SEM. The asterisks indicate significant difference compared with nontg controls (p < 0.05; one-way ANOVA with post hoc Dunnett's).
Figure 8.
Ultrastructural analysis of the neuropathological alterations in MBP hα-syn tg mice. All panels are from the neocortex of 4-month-old tg mice from line 29 imaged by electron microscopy. A, B, Oligodendroglial cells (oligo) within the white matter tracts in the corpus callosum contain fibrillary perinuclear inclusions (arrows), which are surrounded by electrodense material. C, Immunoelectron microscopic analysis of the oligodendroglial cells with an antibody against hα-syn shows abundant gold particles labeling the filaments in the inclusions. D-F, The mitochondria within oligodendroglial cells display abnormal characteristics, including increased size (D), crystalline-like inclusions (arrows; E), irregular crista, and the accumulation of filaments (arrows) around the mitochondria (F). G-I, Mitochondrial alterations in neuronal cells, including formation of crystalline-like inclusions (arrow; G), increased size (H), and irregular crista with electrodense material (I). Scale bars: (in C) A-E, G, H, 10 μm; F, I, 1 μm.
Similar articles
- Oligodendroglial α-synucleinopathy-driven neuroinflammation in multiple system atrophy.
Hoffmann A, Ettle B, Battis K, Reiprich S, Schlachetzki JCM, Masliah E, Wegner M, Kuhlmann T, Riemenschneider MJ, Winkler J. Hoffmann A, et al. Brain Pathol. 2019 May;29(3):380-396. doi: 10.1111/bpa.12678. Epub 2019 Jan 31. Brain Pathol. 2019. PMID: 30444295 Free PMC article. - Neuronal to oligodendroglial α-synuclein redistribution in a double transgenic model of multiple system atrophy.
Rockenstein E, Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Masliah E. Rockenstein E, et al. Neuroreport. 2012 Mar 7;23(4):259-64. doi: 10.1097/WNR.0b013e3283509842. Neuroreport. 2012. PMID: 22314685 Free PMC article. - Human alpha-synuclein overexpressing MBP29 mice mimic functional and structural hallmarks of the cerebellar subtype of multiple system atrophy.
Mészáros L, Riemenschneider MJ, Gassner H, Marxreiter F, von Hörsten S, Hoffmann A, Winkler J. Mészáros L, et al. Acta Neuropathol Commun. 2021 Apr 14;9(1):68. doi: 10.1186/s40478-021-01166-x. Acta Neuropathol Commun. 2021. PMID: 33853667 Free PMC article. - Neuropathology of multiple system atrophy: new thoughts about pathogenesis.
Jellinger KA. Jellinger KA. Mov Disord. 2014 Dec;29(14):1720-41. doi: 10.1002/mds.26052. Epub 2014 Oct 9. Mov Disord. 2014. PMID: 25297524 Review. - The neuropathology, pathophysiology and genetics of multiple system atrophy.
Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. Ahmed Z, et al. Neuropathol Appl Neurobiol. 2012 Feb;38(1):4-24. doi: 10.1111/j.1365-2990.2011.01234.x. Neuropathol Appl Neurobiol. 2012. PMID: 22074330 Review.
Cited by
- α-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy.
Ettle B, Kerman BE, Valera E, Gillmann C, Schlachetzki JC, Reiprich S, Büttner C, Ekici AB, Reis A, Wegner M, Bäuerle T, Riemenschneider MJ, Masliah E, Gage FH, Winkler J. Ettle B, et al. Acta Neuropathol. 2016 Jul;132(1):59-75. doi: 10.1007/s00401-016-1572-y. Epub 2016 Apr 8. Acta Neuropathol. 2016. PMID: 27059609 Free PMC article. - Accumulation of alpha-synuclein within the liver, potential role in the clearance of brain pathology associated with Parkinson's disease.
Reyes JF, Ekmark-Léwen S, Perdiki M, Klingstedt T, Hoffmann A, Wiechec E, Nilsson P, Nilsson KPR, Alafuzoff I, Ingelsson M, Hallbeck M. Reyes JF, et al. Acta Neuropathol Commun. 2021 Mar 20;9(1):46. doi: 10.1186/s40478-021-01136-3. Acta Neuropathol Commun. 2021. PMID: 33743820 Free PMC article. - Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy.
Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, Rockenstein E, May V, Winkler J, Masliah E. Ubhi K, et al. Exp Neurol. 2012 Apr;234(2):405-16. doi: 10.1016/j.expneurol.2012.01.008. Epub 2012 Jan 16. Exp Neurol. 2012. PMID: 22281106 Free PMC article. - In Search of Effective Treatments Targeting α-Synuclein Toxicity in Synucleinopathies: Pros and Cons.
Fouka M, Mavroeidi P, Tsaka G, Xilouri M. Fouka M, et al. Front Cell Dev Biol. 2020 Sep 4;8:559791. doi: 10.3389/fcell.2020.559791. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015057 Free PMC article. Review. - Neuropathology of Multiple System Atrophy, a Glioneuronal Degenerative Disease.
Wakabayashi K, Miki Y, Tanji K, Mori F. Wakabayashi K, et al. Cerebellum. 2024 Feb;23(1):2-12. doi: 10.1007/s12311-022-01407-2. Epub 2022 Apr 26. Cerebellum. 2024. PMID: 35474048
References
- Beal MF (2003) Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann NY Acad Sci 991: 120-131. - PubMed
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301-1306. - PubMed
- Betarbet R, Sherer TB, Greenamyre JT (2002) Animal models of Parkinson's disease. Bio-Essays 24: 308-318. - PubMed
- Clayton D, George J (1998) The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci 21: 249-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG022074/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous