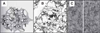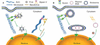Intracellular trafficking of nucleic acids - PubMed (original) (raw)
Review
Intracellular trafficking of nucleic acids
Rui Zhou et al. Expert Opin Drug Deliv. 2004 Nov.
Abstract
Until recently, the attention of most researchers has focused on the first and last steps of gene transfer, namely delivery to the cell and transcription, in order to optimise transfection and gene therapy. However, over the past few years, researchers have realised that the intracellular trafficking of plasmids is more than just a "black box" and is actually one of the major barriers to effective gene delivery. After entering the cytoplasm, following direct delivery or endocytosis, plasmids or other vectors must travel relatively long distances through the mesh of cytoskeletal networks before reaching the nuclear envelope. Once at the nuclear envelope, the DNA must either wait until cell division, or be specifically transported through the nuclear pore complex, in order to reach the nucleoplasm where it can be transcribed. This review focuses on recent developments in the understanding of these intracellular trafficking events as they relate to gene delivery. Hopefully, by continuing to unravel the mechanisms by which plasmids and other gene delivery vectors move throughout the cell, and by understanding the cell biology of gene transfer, superior methods of transfection and gene therapy can be developed.
Figures
Figure 1. Drawings and micrographs demonstrating the cytoplasmic crowding caused by cytoskeletal elements
(A) Drawing from the early 1900’s illustrating the reticular theory of protoplasmic arrangement. Reprinted from [120]. (B) Illustration demonstrating the crowdedness of baker’s yeast cytoplasm (1,000,000 × magnification), with components drawn to scale and at the correct concentrations. Microtubules (large rod in upper left corner), actin (smaller rods running horizontally throughout the panel) and intermediate filaments (medium-sized rod running diagonally along the right hand side) are all represented. Reprinted with permission from The machinery of life. GOODSELL DS, figure 5.2, page 68, (1993), Copyright © Springer-Verlag [9]. (C) High-voltage stereo electron micrographs (80,000 × magnification) depicting the structure of the cytoplasmic matrix in a thin margin of a cultured NRK cell. Reproduced from The Journal of Cell Biology, 1984, vol. 99(1,2), 3s–12s, figure 9, by copyright permission of The Rockefeller University Press [22]. NRK: Normal rat kidney.
Figure 2. Models of cytoplasmic movement of exogenous DNA
(A) Viruses such as adenovirus, HIV, parvovirus and HSV-1 use molecular motors such as the microtubule-based motor dynein to move their genomes toward the host cell’s nucleus, whereas others, such as baculovirus, use the actin cytoskeleton, perhaps through interactions with myosin family members. (B) Plasmid DNA complexed with liposomes is endocytosed and the endosomes are trafficked through interactions between the endosome and dynein, resulting in accumulation at the perinuclear region [26,27]. ‘Naked’ plasmid DNA entering the cytoplasm directly by either electroporation or microinjection is transported to the nucleus using an as yet unidentified pathway.
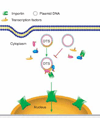
Figure 3. Model of sequence-specific plasmid nuclear import
Plasmids containing a DTS (highlighted in yellow) bind to newly synthesised transcription factors and form a three-dimensional complex in cytoplasm. The nuclear localisation signals on these transcription factors can be recognised by members of the importin family to mediate plasmid nuclear entry. By contrast, plasmids lacking a DTS fail to form import-competent complexes. DTS: DNA nuclear targeting sequence.
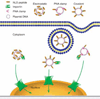
Figure 4. NLS peptide-mediated plasmid nuclear import
Plasmids can be complexed with NLS peptides using different methods, including electrostatic interaction, PNA clamps or covalent conjugation. The various plasmid–NLS peptide complexes can be delivered to and internalised into cells by a variety of methods. Once in the cytoplasm, the NLS peptides complexed or bound to the plasmids can be recognised by the importin family members to facilitate plasmid nuclear entry. NLS: Nuclear localisation signal; PNA: Peptide nucleic acid.
Similar articles
- Nuclear localisation sequence templated nonviral gene delivery vectors: investigation of intracellular trafficking events of LMD and LD vector systems.
Keller M, Harbottle RP, Perouzel E, Colin M, Shah I, Rahim A, Vaysse L, Bergau A, Moritz S, Brahimi-Horn C, Coutelle C, Miller AD. Keller M, et al. Chembiochem. 2003 Apr 4;4(4):286-98. doi: 10.1002/cbic.200390049. Chembiochem. 2003. PMID: 12672108 - Intracellular delivery of nucleic acid by cell-permeable hPP10 peptide.
Ding Y, Zhao X, Geng J, Guo X, Ma J, Wang H, Liu C. Ding Y, et al. J Cell Physiol. 2019 Jul;234(7):11670-11678. doi: 10.1002/jcp.27826. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515802 - On the cellular processing of non-viral nanomedicines for nucleic acid delivery: mechanisms and methods.
Vercauteren D, Rejman J, Martens TF, Demeester J, De Smedt SC, Braeckmans K. Vercauteren D, et al. J Control Release. 2012 Jul 20;161(2):566-81. doi: 10.1016/j.jconrel.2012.05.020. Epub 2012 May 18. J Control Release. 2012. PMID: 22613879 Review. - Nuclear entry of nonviral vectors.
Dean DA, Strong DD, Zimmer WE. Dean DA, et al. Gene Ther. 2005 Jun;12(11):881-90. doi: 10.1038/sj.gt.3302534. Gene Ther. 2005. PMID: 15908994 Free PMC article. Review. - PepFects and NickFects for the Intracellular Delivery of Nucleic Acids.
Arukuusk P, Pärnaste L, Hällbrink M, Langel Ü. Arukuusk P, et al. Methods Mol Biol. 2015;1324:303-15. doi: 10.1007/978-1-4939-2806-4_19. Methods Mol Biol. 2015. PMID: 26202277
Cited by
- Highly acetylated tubulin permits enhanced interactions with and trafficking of plasmids along microtubules.
Badding MA, Dean DA. Badding MA, et al. Gene Ther. 2013 Jun;20(6):616-24. doi: 10.1038/gt.2012.77. Epub 2012 Sep 27. Gene Ther. 2013. PMID: 23013836 Free PMC article. - A novel pathway to detect and cope with exogenous dsDNA.
Kobayashi S, Haraguchi T. Kobayashi S, et al. Commun Integr Biol. 2015 Aug 27;8(5):e1065361. doi: 10.1080/19420889.2015.1065361. eCollection 2015 Sep-Oct. Commun Integr Biol. 2015. PMID: 27064942 Free PMC article. - Co-localization of the amyloid precursor protein and Notch intracellular domains in nuclear transcription factories.
Konietzko U, Goodger ZV, Meyer M, Kohli BM, Bosset J, Lahiri DK, Nitsch RM. Konietzko U, et al. Neurobiol Aging. 2010 Jan;31(1):58-73. doi: 10.1016/j.neurobiolaging.2008.03.001. Epub 2008 Apr 10. Neurobiol Aging. 2010. PMID: 18403052 Free PMC article. - Miniaturization of gene transfection assays in 384- and 1536-well microplates.
Li J, Crowley ST, Duskey J, Khargharia S, Wu M, Rice KG. Li J, et al. Anal Biochem. 2015 Feb 1;470:14-21. doi: 10.1016/j.ab.2014.10.001. Epub 2014 Oct 14. Anal Biochem. 2015. PMID: 25448623 Free PMC article. - BAF is a cytosolic DNA sensor that leads to exogenous DNA avoiding autophagy.
Kobayashi S, Koujin T, Kojidani T, Osakada H, Mori C, Hiraoka Y, Haraguchi T. Kobayashi S, et al. Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):7027-32. doi: 10.1073/pnas.1501235112. Epub 2015 May 19. Proc Natl Acad Sci U S A. 2015. PMID: 25991860 Free PMC article.
References
- Guy J, Drabek D, Antoniou M. Delivery of DNA into mammalian cells by receptor-mediated endocytosis and gene therapy. Mol. Biotechnol. 1995;3(3):237–248. - PubMed
- Bally MB, Harvie P, Wong FM, et al. Biological barriers to cellular delivery of lipid-based DNA carriers. Adv. Drug Deliv. Rev. 1999;38(3):291–315. - PubMed
- Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochemistry and Bioenergetics. 1996;41(2):135–160.
- Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9(24):1647–1652. - PubMed
- Lechardeur D, Lukacs GL. Intracellular barriers to non-viral gene transfer. Curr. Gene Ther. 2002;2(2):183–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL071643-020005/HL/NHLBI NIH HHS/United States
- R01 HL059956-06A2/HL/NHLBI NIH HHS/United States
- R01 EY012962/EY/NEI NIH HHS/United States
- R01 HL059956/HL/NHLBI NIH HHS/United States
- P01 HL071643/HL/NHLBI NIH HHS/United States
- R01 EY012962-04/EY/NEI NIH HHS/United States
- EY 12962/EY/NEI NIH HHS/United States
- HL 59956/HL/NHLBI NIH HHS/United States
- HL 76139/HL/NHLBI NIH HHS/United States
- HL 71643/HL/NHLBI NIH HHS/United States
- T32 HL076139/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous