Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy - PubMed (original) (raw)
Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy
Casey L Moulson et al. J Invest Dermatol. 2005 Nov.
Abstract
Restrictive dermopathy (RD) is a lethal human genetic disorder characterized by very tight, thin, easily eroded skin, rocker bottom feet, and joint contractures. This disease was recently reported to be associated with a single heterozygous mutation in ZMPSTE24 and hypothesized to be a digenic disorder (Navarro et al, Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet 13:2493-2503, 2004). ZMPSTE24 encodes an enzyme necessary for the correct processing and maturation of lamin A, an intermediate filament component of the nuclear envelope. Here we present four unrelated patients with homozygous mutations in ZMPSTE24 and a fifth patient with compound heterozygous mutations in ZMPSTE24. Two of the three different mutations we found are novel, and all are single base insertions that result in messenger RNA frameshifts. As a consequence of the presumed lack of ZMPSTE24 activity, prelamin A, the unprocessed toxic form of lamin A, was detected in the nuclei of both cultured cells and tissue from RD patients, but not in control nuclei. Abnormally aggregated lamin A/C was also observed. These results indicate that RD is an autosomal recessive laminopathy caused by inactivating ZMPSTE24 mutations that result in defective processing and nuclear accumulation of prelamin A.
Figures
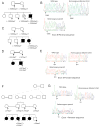
Figure 1. Family pedigrees, ZMPSTE24 genotypes, and sequence chromatograms.
Arrows indicate individuals whose DNA was tested. ZMPSTE24 mutations are shown below the pedigree symbols. (A) Pedigree of a consanguineous Dutch family; the father’s maternal grandmother and the mother’s paternal grandmother were sisters. The affected child had a homozygous thymidine duplication in ZMPSTE24 exon 9. (B) Sequence chromatograms of exon 9 (reverse direction) show a duplicated thymidine in a stretch of nine. Overlap of the normal and mutated strand sequences occurred after the insertion in this and in all the other heterozygous samples described below. (C) Pedigree of a nonconsanguineous American family carrying the same mutation as the Dutch family. (D) Pedigree from a consanguineous Guatemalan family; the father’s paternal grandfather was a paternal uncle of the mother. (E) Sequence chromatograms of exon 5 (forward direction) showing the duplicated thymidine in a stretch of seven. (F) Five affected children were born to two pairs of related parents from a Mennonite kindred; the fathers were brothers of each other, and the mothers were first cousins of each other. (G) Sequence chromatograms of ZMPSTE24 exon 1 (reverse direction) show an extra thymidine homozygous in the affected child.
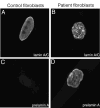
Figure 2. Immunofluorescent localization of lamins A and C and prelamin A in control and RD patient fibroblasts.
(A) Confocal micrographs show localization of lamins A and C in nuclei of a control fibroblast and (B) a fibroblast from a RD patient. (C) Confocal micrographs show staining for prelamin A in a control fibroblast and (D) in a fibroblast from a RD patient.
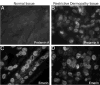
Figure 3. Immunofluorescent localization of prelamin A and emerin in control tissue and tissue from a patient with RD.
(A) Prelamin A was undetectable in a kidney section from a normal patient but clearly present in nuclei in a liver section from a RD patient (B). (C) Nuclei in control cells and (D) in the RD patient’s cells are labeled with anti-emerin to show the presence of nuclear envelopes.
Comment in
- Lamin processing comes of age.
van Steensel MA, Frank J. van Steensel MA, et al. J Invest Dermatol. 2005 Nov;125(5):xii-xiii. doi: 10.1111/j.0022-202X.2005.23871.x. J Invest Dermatol. 2005. PMID: 16297177 No abstract available.
Similar articles
- Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy.
Navarro CL, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Geneviève D, Hadj-Rabia S, Gaudy-Marqueste C, Smitt HS, Vabres P, Faivre L, Verloes A, Van Essen T, Flori E, Hennekam R, Beemer FA, Laurent N, Le Merrer M, Cau P, Lévy N. Navarro CL, et al. Hum Mol Genet. 2004 Oct 15;13(20):2493-503. doi: 10.1093/hmg/ddh265. Epub 2004 Aug 18. Hum Mol Genet. 2004. PMID: 15317753 - Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors.
Navarro CL, Cadiñanos J, De Sandre-Giovannoli A, Bernard R, Courrier S, Boccaccio I, Boyer A, Kleijer WJ, Wagner A, Giuliano F, Beemer FA, Freije JM, Cau P, Hennekam RC, López-Otín C, Badens C, Lévy N. Navarro CL, et al. Hum Mol Genet. 2005 Jun 1;14(11):1503-13. doi: 10.1093/hmg/ddi159. Epub 2005 Apr 20. Hum Mol Genet. 2005. PMID: 15843403 - A homozygous ZMPSTE24 null mutation in combination with a heterozygous mutation in the LMNA gene causes Hutchinson-Gilford progeria syndrome (HGPS): insights into the pathophysiology of HGPS.
Denecke J, Brune T, Feldhaus T, Robenek H, Kranz C, Auchus RJ, Agarwal AK, Marquardt T. Denecke J, et al. Hum Mutat. 2006 Jun;27(6):524-31. doi: 10.1002/humu.20315. Hum Mutat. 2006. PMID: 16671095 - A humanized yeast system to analyze cleavage of prelamin A by ZMPSTE24.
Spear ED, Alford RF, Babatz TD, Wood KM, Mossberg OW, Odinammadu K, Shilagardi K, Gray JJ, Michaelis S. Spear ED, et al. Methods. 2019 Mar 15;157:47-55. doi: 10.1016/j.ymeth.2019.01.001. Epub 2019 Jan 6. Methods. 2019. PMID: 30625386 Free PMC article. Review. - [A-type lamins and progeroïd syndromes : persistent farnesylation with dramatic effects].
Navarro CL, Poitelon Y, Lévy N. Navarro CL, et al. Med Sci (Paris). 2008 Oct;24(10):833-40. doi: 10.1051/medsci/20082410833. Med Sci (Paris). 2008. PMID: 18950579 Review. French.
Cited by
- Defective prelamin A processing promotes unconventional necroptosis driven by nuclear RIPK1.
Yang Y, Zhang J, Lv M, Cui N, Shan B, Sun Q, Yan L, Zhang M, Zou C, Yuan J, Xu D. Yang Y, et al. Nat Cell Biol. 2024 Apr;26(4):567-580. doi: 10.1038/s41556-024-01374-2. Epub 2024 Mar 27. Nat Cell Biol. 2024. PMID: 38538837 - The farnesyl transferase inhibitor (FTI) lonafarnib improves nuclear morphology in ZMPSTE24-deficient fibroblasts from patients with the progeroid disorder MAD-B.
Odinammadu KO, Shilagardi K, Tuminelli K, Judge DP, Gordon LB, Michaelis S. Odinammadu KO, et al. Nucleus. 2023 Dec;14(1):2288476. doi: 10.1080/19491034.2023.2288476. Epub 2023 Dec 5. Nucleus. 2023. PMID: 38050983 Free PMC article. - Prelamin A and ZMPSTE24 in premature and physiological aging.
Worman HJ, Michaelis S. Worman HJ, et al. Nucleus. 2023 Dec;14(1):2270345. doi: 10.1080/19491034.2023.2270345. Epub 2023 Oct 26. Nucleus. 2023. PMID: 37885131 Free PMC article. Review. - Hereditary severe insulin resistance syndrome: Pathogenesis, pathophysiology, and clinical management.
Iqbal J, Jiang HL, Wu HX, Li L, Zhou YH, Hu N, Xiao F, Wang T, Xu SN, Zhou HD. Iqbal J, et al. Genes Dis. 2022 Apr 11;10(5):1846-1856. doi: 10.1016/j.gendis.2022.03.016. eCollection 2023 Sep. Genes Dis. 2022. PMID: 37492723 Free PMC article. Review. - "Bone-SASP" in Skeletal Aging.
Fang CL, Liu B, Wan M. Fang CL, et al. Calcif Tissue Int. 2023 Jul;113(1):68-82. doi: 10.1007/s00223-023-01100-4. Epub 2023 May 31. Calcif Tissue Int. 2023. PMID: 37256358 Free PMC article. Review.
References
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. - PubMed
- Capanni C, Cenni V, Mattioli E, et al. Failure of lamin A/C to functionally assemble in R482L mutated familial partial lipodystrophy fibroblasts: altered intermolecular interaction with emerin and implications for gene transcription. Exp Cell Res. 2003;291:122–134. - PubMed
- Caux F, Dubosclard E, Lascols O, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88:1006–1013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous