Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism - PubMed (original) (raw)
Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism
Parvin Hakimi et al. Nutr Metab (Lond). 2005.
Abstract
Background: The metabolic function of PEPCK-C is not fully understood; deletion of the gene for the enzyme in mice provides an opportunity to fully assess its function.
Methods: The gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) (EC 4.1.1.32) (PEPCK-C) was deleted in mice by homologous recombination (PEPCK-C-/- mice) and the metabolic consequences assessed.
Results: PEPCK-C-/- mice became severely hypoglycemic by day two after birth and then died with profound hypoglycemia (12 mg/dl). The mice had milk in their stomachs at day two after birth and the administration of glucose raised the concentration of blood glucose in the mice but did not result in an increased survival. PEPCK-C-/- mice have two to three times the hepatic triglyceride content as control littermates on the second day after birth. These mice also had an elevation of lactate (2.5 times), beta-hydroxybutyrate (3 times) and triglyceride (50%) in their blood, as compared to control animals. On day two after birth, alanine, glycine, glutamine, glutamate, aspartate and asparagine were elevated in the blood of the PEPCK-C-/- mice and the blood urea nitrogen concentration was increased by 2-fold. The rate of oxidation of [2-14C]-acetate, and [5-14C]-glutamate to 14CO2 by liver slices from PEPCK-C-/- mice at two days of age was greatly reduced, as was the rate of fatty acid synthesis from acetate and glucose. As predicted by the lack of PEPCK-C, the concentration of malate in the livers of the PEPCK-C-/- mice was 10 times that of controls.
Conclusion: We conclude that PEPCK-C is required not only for gluconeogenesis and glyceroneogenesis but also for cataplerosis (i.e. the removal of citric acid cycle anions) and that the failure of this process in the livers of PEPCK-C-/- mice results in a marked reduction in citric acid cycle flux and the shunting of hepatic lipid into triglyceride, resulting in a fatty liver.
Figures
Figure 1
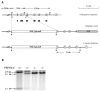
Generation of PEPCK-C-/- mice. Panel A. Diagram of the targeting vector, which is aligned with endogenous sequence of PEPCK-C gene. The sequence between Pst I and Xho I, which covers part of the gene promoter and the exons 1 and 2 in endogenous gene, was replaced with Neo resistance gene (PGK_βgal-neoR) in the targeting vector. H, Hind III; P, Pst I; X, Xho I. PGK_βgal-neoR, the phosphoglycerate kinase gene promoter drives a fusion gene of βgal and neoR. DTA, the diphtheria toxin A chain. Panel B. Genotyping of the PEPCK-C-/- mice by Southern blotting. Genomic DNA was digested with Hind III and hybridized with a PEPCK-C cDNA probe composed of exons 1~6 of the rat PEPCK-C gene (black bars). For the wild type allele of PEPCK-C, 1.3-kb and 2.75-kb fragments were detected; only one fragment (2.5 kb) was excepted for the targeted allele.
Figure 2
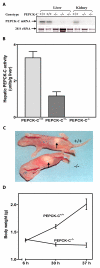
Characterization of the PEPCK-C-/- mice. Panel A. A representative Northern blot for mRNA isolated from the liver and kidney of two-day-old mice. Panel B. PEPCK-C activity was determined in the livers of two-day-old mice. The results are expressed as the mean ± S.E. for three animals in each group. Panel C. Photograph of two-day-old PEPCK-C-/- mice. Panel D. Growth retardation of PEPCK-C-/- mice. Body weights of neonates were measure at 6, 30, and 37 h after birth. Three wild type mice and three PEPCK-C-/- mice were used.
Figure 3
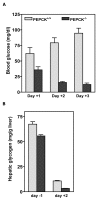
Alterations in glucose homeostaisis in PEPCK-C-/- and control mice during the perinatal period. Panel A Hypoglycemia in PEPCK-C-/- mice. The concentration of blood glucose was measured in mice at one, two and three days after birth. The results are expressed as the mean ± S.E. for from three to six animals. Panel B. Increased mobilization of hepatic glycogen. The hepatic glycogen level was analyzed at the age of fetal day 19 (day -1) and neonatal day (day +2). The results are expressed as the mean ± S.E. for three animals in each group.
Figure 4
Morphology of the livers of PEPCK-C-/- and control mice. Livers of two-day-old pups were analyzed with H&E staining. The arrow indicates fat accumulation in the liver of PEPCK-C-/- mice.
Figure 5
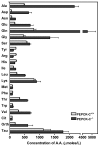
The concentration of amino acids in the blood of PEPCK-C-/- mice and controls. The levels of amino acids were determined in mice at two days after birth. The results are expressed as the mean ± S.E. of three controls and four PEPCK-C-/- mice.
Figure 6
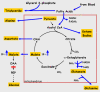
The role of PEPCK-C in cataplerosis in the liver. The reactions of the citric acid cycle are presented, with the major end-products shown in gold boxes; the large red box represents the mitochondrial membrane. The concentrations of amino acids, β-hydroxybutyrate, glucose and triglyceride were determined from blood samples, while the levels of malate and pyruvate were determined from freeze-clamped liver. The ablation of PEPCK-C (shown by a red bar) results in a 10-fold increase in the concentration of malate in the liver (we did not distinguish between malate in the cytosol and the mitochondria) and a build-up of cycle intermediates [23]. This leads to a decrease in the rate of citric acid cycle flux and the resultant accumulation of acetyl CoA, which is subsequently converted to ketone bodies and released by the liver. The rate of fatty acid oxidation in the PEPCK-C-/- mice is also markedly decreased, resulting in an increase in triglyceride synthesis from these fatty acids that leads to the development of a fatty liver. There is also a marked increase in the concentration of amino acids in the blood that were generated from citric acid cycle intermediates. The increased rate of flux of intermediates leaving the citric acid cycle is denoted by heavy arrows.
Similar articles
- PCK1 and PCK2 as candidate diabetes and obesity genes.
Beale EG, Harvey BJ, Forest C. Beale EG, et al. Cell Biochem Biophys. 2007;48(2-3):89-95. doi: 10.1007/s12013-007-0025-6. Cell Biochem Biophys. 2007. PMID: 17709878 Review. - Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase.
Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Burgess SC, et al. J Biol Chem. 2004 Nov 19;279(47):48941-9. doi: 10.1074/jbc.M407120200. Epub 2004 Sep 3. J Biol Chem. 2004. PMID: 15347677 - Cytosolic phosphoenolpyruvate carboxykinase as a cataplerotic pathway in the small intestine.
Potts A, Uchida A, Deja S, Berglund ED, Kucejova B, Duarte JA, Fu X, Browning JD, Magnuson MA, Burgess SC. Potts A, et al. Am J Physiol Gastrointest Liver Physiol. 2018 Aug 1;315(2):G249-G258. doi: 10.1152/ajpgi.00039.2018. Epub 2018 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29631378 Free PMC article. - Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver.
Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Burgess SC, et al. Cell Metab. 2007 Apr;5(4):313-20. doi: 10.1016/j.cmet.2007.03.004. Cell Metab. 2007. PMID: 17403375 Free PMC article. - Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression.
Hanson RW, Reshef L. Hanson RW, et al. Annu Rev Biochem. 1997;66:581-611. doi: 10.1146/annurev.biochem.66.1.581. Annu Rev Biochem. 1997. PMID: 9242918 Review.
Cited by
- Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice.
Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Burgess SC, et al. J Biol Chem. 2006 Jul 14;281(28):19000-8. doi: 10.1074/jbc.M600050200. Epub 2006 May 2. J Biol Chem. 2006. PMID: 16670093 Free PMC article. - Porcine adiponectin receptor 1 transgene resists high-fat/sucrose diet-induced weight gain, hepatosteatosis and insulin resistance in mice.
Liu BH, Lin YY, Wang YC, Huang CW, Chen CC, Wu SC, Mersmann HJ, Cheng WT, Ding ST. Liu BH, et al. Exp Anim. 2013;62(4):347-60. doi: 10.1538/expanim.62.347. Exp Anim. 2013. PMID: 24172199 Free PMC article. - Liver Glucokinase(A456V) Induces Potent Hypoglycemia without Dyslipidemia through a Paradoxical Induction of the Catalytic Subunit of Glucose-6-Phosphatase.
Vidal-Alabró A, Gómez-Valadés AG, Méndez-Lucas A, Llorens J, Bartrons R, Bermúdez J, Perales JC. Vidal-Alabró A, et al. Int J Endocrinol. 2011;2011:707928. doi: 10.1155/2011/707928. Epub 2011 Dec 13. Int J Endocrinol. 2011. PMID: 22194744 Free PMC article. - Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and mechanisms beyond gluconeogenesis.
Yu S, Meng S, Xiang M, Ma H. Yu S, et al. Mol Metab. 2021 Nov;53:101257. doi: 10.1016/j.molmet.2021.101257. Epub 2021 May 18. Mol Metab. 2021. PMID: 34020084 Free PMC article. Review. - Dysregulation of Lipid Metabolism in Mkp-1 Deficient Mice during Gram-Negative Sepsis.
Li J, Wang X, Ackerman WE 4th, Batty AJ, Kirk SG, White WM, Wang X, Anastasakis D, Samavati L, Buhimschi I, Nelin LD, Hafner M, Liu Y. Li J, et al. Int J Mol Sci. 2018 Dec 6;19(12):3904. doi: 10.3390/ijms19123904. Int J Mol Sci. 2018. PMID: 30563203 Free PMC article.
References
- Hanson RW, Patel YM. P-enolpyruvate carboxykinase: the gene and the enzyme. In: Meister A, editor. Advances in Enzymology. Vol. 69. New York, John Wiley and Sons; 1994. pp. 203–281. - PubMed
- Reshef L, Hanson RW, Ballard FJ. A possible physiological role for glyceroneogenesis in rat adipose tissue. J Biol Chem. 1970;245:5979–5984. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials