Coordinated transport of phosphorylated amyloid-beta precursor protein and c-Jun NH2-terminal kinase-interacting protein-1 - PubMed (original) (raw)
Coordinated transport of phosphorylated amyloid-beta precursor protein and c-Jun NH2-terminal kinase-interacting protein-1
Zoia Muresan et al. J Cell Biol. 2005.
Abstract
The transmembrane protein amyloid-beta precursor protein (APP) and the vesicle-associated protein c-Jun NH(2)-terminal kinase-interacting protein-1 (JIP-1) are transported into axons by kinesin-1. Both proteins may bind to kinesin-1 directly and can be transported separately. Because JIP-1 and APP can interact, kinesin-1 may recruit them as a complex, enabling their cotransport. In this study, we tested whether APP and JIP-1 are transported together or separately on different vesicles. We found that, within the cellular context, JIP-1 preferentially interacts with Thr(668)-phosphorylated APP (pAPP), compared with nonphosphorylated APP. In neurons, JIP-1 colocalizes with vesicles containing pAPP and is excluded from those containing nonphosphorylated APP. The accumulation of JIP-1 and pAPP in neurites requires kinesin-1, and the expression of a phosphomimetic APP mutant increases JIP-1 transport. Down-regulation of JIP-1 by small interfering RNA specifically impairs transport of pAPP, with no effect on the trafficking of nonphosphorylated APP. These results indicate that the phosphorylation of APP regulates the formation of a pAPP-JIP-1 complex that accumulates in neurites independent of nonphosphorylated APP.
Figures
Figure 1.
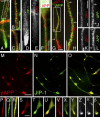
Colocalization of JIP-1 with pAPP in neuronal processes. CAD cells (A–F and M–Y) and cortical neurons (G–L, Z–β) were double labeled for JIP-1 and either total APP (antibody 4G8; A–L) or pAPP (M–β). (A–L) The majority of total APP (A–F, red; G–L, green) is present in vesicles distinct from those carrying JIP-1 (A–F, green; G–L, red). (B and H) Enlargements of the boxed areas in A and G. (D and E) Single color enlargements of the process terminal shown in A. Similarly, I–L represent single color enlargements of regions from the distal and proximal neurite shown in G and H to reveal the distinct distribution of JIP-1 and APP along the neurite. (G, H, J, and L) Arrows mark the ends of corresponding segments. (M–β) JIP-1 (M–Y and β, green; α, white) colocalizes extensively with pAPP (M–Y and β, red; Z, white) throughout the processes (P–R) and at their terminals (S–β). Bars: (A–L) 5 μm; (M–O) 20 μm; (P–β) 10 μm.
Figure 2.
Biochemical analysis of JIP-1 interaction with nonphosphorylated and phosphorylated APP. (A) SDS-PAGE separation of synthetic, biotinylated, phosphorylated (pAPPCD), and nonphosphorylated (APPCD) polypeptides encompassing the APP cytoplasmic domain (Tris-tricine gel). Peptides were detected on the same gel, but the lanes are presented in separate boxes to indicate that the contrast was adjusted differently for each lane. This was done to demonstrate the purity of the peptides rather than their relative amounts. (B) Pull-down experiment done with the polypeptides shown in A. Equal amounts of the two polypeptides (Ponceau S) were incubated with lysates of COS-1 cells transfected with FLAG-tagged JIP-1 or JIP-2 and collected on streptavidin-Sepharose. Copurified JIPs were detected by immunoblotting with antibodies to the FLAG tag (marked JIP-1 and JIP-2). Note that JIP-1, but not JIP-2, binds preferentially to pAPPCD. Densitometric analysis of blots showed that JIP-1 binding to APPCD is 30–40% lower than to pAPPCD. A control lane (Beads) shows residual binding of JIP-1–FLAG to beads in the absence of added polypeptide. The anti-pAPP antibody detects the phosphorylated, but not the nonphosphorylated, polypeptide in the pulled-down material (Anti-pAPP). (C) Differential binding of JIP-1 and Fe65 to phosphorylated and nonphosphorylated APP polypeptides (ELISA). Streptavidin-coated ELISA plates, preadsorbed with biotinylated APPCD or pAPPCD, were incubated with increasing concentrations of cytosol (expressed as percentages of the total incubation mixture; see Materials and methods) from HEK293 cells transfected with either JIP-1–FLAG or Fe65-myc. Bound proteins were detected colorimetrically, using alkaline phosphatase–labeled antibodies to the tags. Error bars represent SEM (n = 3). (D) Coprecipitation of phosphorylated and nonphosphorylated APP with JIP-1. CAD cells were transfected separately with human APP695 and with JIP-1–FLAG; the two cell extracts were mixed and incubated overnight. Mixtures were immunoprecipitated with an anti-FLAG antibody, and immunoprecipitated material was analyzed by immunoblotting with antibodies to total APP, pAPP, and FLAG. The three polypeptides detected with the anti-APP antibody correspond to immature and mature forms of APP695 (arrow points to the fully glycosylated form of APP). (right, No JIP-1) Nonspecific binding of APP and pAPP to beads, in the absence of JIP-1–FLAG-containing cytosol, was low.
Figure 3.
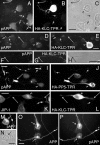
Localization of pAPP at neurite terminals requires kinesin-1. (A–L) Localization of pAPP (A–J) and JIP-1 (K and L) in CAD cells overexpressing the tagged, kinesin-1 dominant-negative construct, HA-KLC-TPR (A–H, K, and L), or the control construct, HA-PP5-TPR (I and J). Two examples show the effect of HA-KLC-TPR expression on pAPP localization (A–C and D–H). Note that pAPP and JIP-1 accumulate in the cell body of cells transfected with HA-KLC-TPR (arrowheads) and are not detected at the terminals of their processes (long arrows). In contrast, pAPP and JIP-1 localize to the neurite terminals in nontransfected cells (short arrows). Localization of pAPP is not affected in cells transfected with HA-PP5-TPR (compare short with long arrows in I and J). No pAPP accumulation in the cell body of the transfected cell is seen (I and J, arrowheads). (F–H) Enlarged images of the neurite shown in D and E. (M–P) pAPP can be detected in the Golgi area in CAD cells. (M and N) Vesicular localization of endogenous pAPP in the Golgi region (arrow) of a CAD cell. (O and P) In cells transfected with APP695, pAPP is found throughout the processes and in the cell body (P, arrows). (O) Image depicts total APP. (C, H, and N) Phase-contrast micrographs. Bars, 20 μm.
Figure 4.
JIP-1 facilitates the binding of kinesin-1 to pAPP and the transport of pAPP. (A) Coprecipitation of kinesin-1 with constitutively phosphorylated, but not nonphosphorylatable APP, is enhanced by JIP-1. Lysates of CAD cells transfected with APP-Thr668Glu (APP(T-E)) or APP-Thr668Ala (APP(T-A)) were mixed with lysates of CAD cells transfected with JIP-1–FLAG (+) or of nontransfected (−) CAD cells. Squid kinesin-1 was added to the mixtures, and APP was immunoprecipitated with an antibody to human APP (mAbP2-1). Immunoprecipitated material was tested for the presence of KHC and KLC by immunoblotting. APP blots showed equal amounts of immunoprecipitated APP. Densitometric analysis of blots showed that, in the case of APP-Thr668Glu, approximately three times more kinesin-1 is coprecipitated from mixtures that contained JIP-1 (+). (B–K) CAD cells transfected with FLAG-tagged JIP-1 (B–H) or myc-tagged Fe65 (I–K) were double labeled for endogenous pAPP (B, E, G, and I) and the tag (C, F, H, and J). Note that the cells expressing JIP-1–FLAG show an increased amount of pAPP at the process terminals, which colocalizes with the expressed JIP-1 (B–H, long arrows). In contrast, pAPP does not colocalize with Fe65-myc (I–K). (B, E, and D) Short arrows point to the terminals of nontransfected cells. (L–V) Constitutively phosphorylated APP (APP-Thr668Glu), but not nonphosphorylatable APP (APP-Thr668Ala), promotes a more efficient transport of endogenous JIP-1 into CAD cell processes. Images show the localization of endogenous JIP-1 and exogenously expressed APP in CAD cells transfected with human wild-type APP (APPwt), APP-Thr668Ala, and APP-Thr668Glu. APP was detected with an antibody to the human protein that minimally cross-reacts with mouse APP (6E10). Note the increased presence of JIP-1 at the terminals of the cells transfected with APP-Thr668Glu (L–O, long arrows), but not APP–Thr668Ala (P–R, arrows). Short arrows in (L and M) point to the terminals of nontransfected cells. (V) Additional examples of immunolabeled neurite terminals of transfected cells, shown at high magnification. Bars: (B–H) and (L–U) 40 μm; (I–K) 20 μm; (V) 10 μm.
Figure 5.
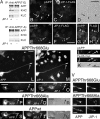
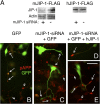
JIP-1 is required for the accumulation of pAPP in neurites. (A–G) Down-regulation of JIP-1 by siRNA. CAD cells were transfected with GFP cDNA with or without JIP-1–specific siRNA and analyzed by immunoblotting (A) and immunocytochemistry (B–G) for JIP-1 expression. Transfers of cell lysates were probed with anti–JIP-1 antibody, which detects one polypeptide in CAD cells (A). (A) Before blotting, transfers were stained with Ponceau S to compare protein loads. Molecular size markers are in kilodaltons. In immunofluorescence, cells transfected with GFP alone (control) accumulated JIP-1 at terminals (B–D; long arrows in D), similar to nontransfected cells (D, short arrows). No JIP-1 is detected at neurite endings (E–G, long arrows) in cells cotransfected with GFP and JIP-1–specific siRNA. (E–G) Short arrows point to terminals of nontransfected cells. Only the left portion of the image in D is shown in B and C. (B–D) The asterisks mark corresponding regions. (H–M) Down-regulation of JIP-1 leads to diminished accumulation of pAPP (J and K) but not total, mostly nonphosphorylated APP (L and M) in neurites. CAD cells were transfected with GFP alone (H and I; control) or with GFP and JIP-1–specific siRNA (J–M). Note that down-regulation of JIP-1 occasionally leads to accumulation of pAPP in the cell body (J and K, arrowheads in insets), likely because of impaired pAPP transport. pAPP localizes normally, at neurite endings, in cells transfected with GFP alone (H and I). Arrows point to process terminals. Bars, 20 μm.
Figure 6.
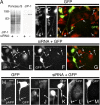
Complementation of JIP-1–deficient CAD cells with human JIP-1 restores pAPP transport into neurites. (A) Down-regulation of mouse JIP-1 (mJIP-1) but not human JIP-1 (hJIP-1) by mJIP-1–specific siRNA. CAD cells were transfected with FLAG-tagged mJIP-1 or hJIP-1 cDNA with or without mJIP-1–specific siRNA. Cell lysates were analyzed for expression of exogenous JIP-1 by immunoblotting with anti-FLAG antibody. The actin blot shows equal protein load. Note that mJIP-1–specific siRNA does not down-regulate hJIP-1 (right). (B–E) Rescue of pAPP transport by hJIP-1 in mJIP-1–deficient cells. CAD cells were transfected with GFP (B), GFP and mJIP-1–specific siRNA (C), or GFP, mJIP-1–specific siRNA, and hJIP-1–FLAG (D and E) and analyzed by immunocytochemistry for pAPP. Note that transfection with GFP alone does not affect localization of pAPP (B), whereas cotransfection with mJIP-1–specific siRNA eliminates accumulation of pAPP at neurite terminals (C). However, accumulation of pAPP at neurite endings is restored in cells transfected with hJIP-1 in addition to GFP and mJIP-1–specific siRNA (D and E). Long and short arrows point to the terminals of transfected and nontransfected cells, respectively. Bar, 50 μm.
Figure 7.
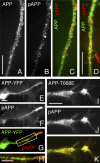
Nonphosphorylated APP is largely segregated from pAPP during transport. (A–D) Double labeling of endogenous, total APP (antibody 4G8; green) and pAPP (red) in CAD cells (A–C) and cortical neurons (D). Note that the antibody to total APP largely labels vesicles that do not stain for pAPP. The CAD cells shown in E–H and I–K have been transfected with APP-YFP (wild type) and nontagged APP-Thr668Glu (APP-T668E), respectively. Antibodies to total APP and pAPP label the same vesicle population in cells transfected with APP-Thr668Glu, where the constitutively phosphorylated APP predominates (I–K). This situation differs from the endogenous condition (nontransfected cells; A–D) or APP-YFP transfected cells (E–H), where only a fraction of APP is phosphorylated and sorted away from nonphosphorylated APP into distinct transport vesicles. Note that the antibody to pAPP cross-reacts with APP-Thr668Glu (I–K). H is an enlargement of the boxed area in G. Bars: (A–H) 10 μm; (I–K) 5 μm.
Figure 8.
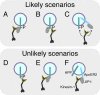
Schematic representation of cotransport of JIP-1 and pAPP. The diagram shows likely (i.e., experimentally supported; A–C) and unlikely (D–F) scenarios for transport of JIP-1, pAPP, and nonphosphorylated APP. (C) Kinesin-1 may bind to either pAPP or JIP-1. In vivo, pAPP is not transported without JIP-1 (D), and JIP-1 is not cotransported with nonphosphorylated APP (E and F).
Similar articles
- c-Jun NH2-terminal kinase-interacting protein-3 facilitates phosphorylation and controls localization of amyloid-beta precursor protein.
Muresan Z, Muresan V. Muresan Z, et al. J Neurosci. 2005 Apr 13;25(15):3741-51. doi: 10.1523/JNEUROSCI.0152-05.2005. J Neurosci. 2005. PMID: 15829626 Free PMC article. - c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer's amyloid precursor protein with JNK.
Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, Kouyama K, Yamamoto T, Kyriakis JM, Nishimoto I. Matsuda S, et al. J Neurosci. 2001 Sep 1;21(17):6597-607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. J Neurosci. 2001. PMID: 11517249 Free PMC article. - The cytoplasmic region of the amyloid β-protein precursor (APP) is necessary and sufficient for the enhanced fast velocity of APP transport by kinesin-1.
Tsukamoto M, Chiba K, Sobu Y, Shiraki Y, Okumura Y, Hata S, Kitamura A, Nakaya T, Uchida S, Kinjo M, Taru H, Suzuki T. Tsukamoto M, et al. FEBS Lett. 2018 Aug;592(16):2716-2724. doi: 10.1002/1873-3468.13204. Epub 2018 Aug 12. FEBS Lett. 2018. PMID: 30055048 - Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease.
Kins S, Lauther N, Szodorai A, Beyreuther K. Kins S, et al. Neurodegener Dis. 2006;3(4-5):218-26. doi: 10.1159/000095259. Neurodegener Dis. 2006. PMID: 17047360 Review. - Adaptor protein interactions: modulators of amyloid precursor protein metabolism and Alzheimer's disease risk?
King GD, Scott Turner R. King GD, et al. Exp Neurol. 2004 Feb;185(2):208-19. doi: 10.1016/j.expneurol.2003.10.011. Exp Neurol. 2004. PMID: 14736502 Review.
Cited by
- The amyloid precursor protein: beyond amyloid.
Zheng H, Koo EH. Zheng H, et al. Mol Neurodegener. 2006 Jul 3;1:5. doi: 10.1186/1750-1326-1-5. Mol Neurodegener. 2006. PMID: 16930452 Free PMC article. - Amyloid-β precursor protein: Multiple fragments, numerous transport routes and mechanisms.
Muresan V, Ladescu Muresan Z. Muresan V, et al. Exp Cell Res. 2015 May 15;334(1):45-53. doi: 10.1016/j.yexcr.2014.12.014. Epub 2015 Jan 6. Exp Cell Res. 2015. PMID: 25573596 Free PMC article. Review. - A Role for Drosophila Amyloid Precursor Protein in Retrograde Trafficking of L1-Type Cell Adhesion Molecule Neuroglian.
Penserga T, Kudumala SR, Poulos R, Godenschwege TA. Penserga T, et al. Front Cell Neurosci. 2019 Jul 12;13:322. doi: 10.3389/fncel.2019.00322. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31354437 Free PMC article. - Spatial and temporal characteristics of normal and perturbed vesicle transport.
Iacobucci GJ, Rahman NA, Valtueña AA, Nayak TK, Gunawardena S. Iacobucci GJ, et al. PLoS One. 2014 May 30;9(5):e97237. doi: 10.1371/journal.pone.0097237. eCollection 2014. PLoS One. 2014. PMID: 24878565 Free PMC article. - Potential role of PCTAIRE-2, PCTAIRE-3 and P-Histone H4 in amyloid precursor protein-dependent Alzheimer pathology.
Chaput D, Kirouac L, Stevens SM Jr, Padmanabhan J. Chaput D, et al. Oncotarget. 2016 Feb 23;7(8):8481-97. doi: 10.18632/oncotarget.7380. Oncotarget. 2016. PMID: 26885753 Free PMC article.
References
- Ando, K., K.I. Iijima, J.I. Elliott, Y. Kirino, and T. Suzuki. 2001. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J. Biol. Chem. 276:40353–40361. - PubMed
- Cole, D.G., S.W. Chinn, K.P. Wedaman, K. Hall, T. Vuong, and J.M. Scholey. 1993. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature. 366:268–270. - PubMed
- Iijima, K., K. Ando, S. Takeda, Y. Satoh, T. Seki, S. Itohara, P. Greengard, Y. Kirino, A.C. Nairn, and T. Suzuki. 2000. Neuron-specific phosphorylation of Alzheimer's beta-amyloid precursor protein by cyclin-dependent kinase 5. J. Neurochem. 75:1085–1091. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous