BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway - PubMed (original) (raw)
BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway
Andrew Craxton et al. J Exp Med. 2005.
Abstract
The B cell activating factor belonging to the tumor necrosis factor family (BAFF) is required for B cell survival and maturation. The mechanisms by which BAFF mediates B cell survival are less understood. We found that BAFF and a proliferation-inducing ligand (APRIL), which are related, block B cell antigen receptor (BCR)-induced apoptosis upstream of mitochondrial damage, which is consistent with a role for Bcl-2 family proteins. BCR ligation strongly increased expression of the proapoptotic Bcl-2 homology 3-only Bcl-2 protein Bim in both WEHI-231 and splenic B cells, and increases in Bim were reversed by BAFF or APRIL. Small interfering RNA vector-mediated suppression of Bim blocked BCR-induced apoptosis. BAFF also induced Bim phosphorylation and inhibited BCR-induced association of Bim with Bcl-2. BAFF induced delayed but sustained stimulation of extracellular signal-regulated kinase (ERK) and its activators, mitogen-activated protein kinase/ERK activating kinase (MEK) and c-Raf, and MEK inhibitors promoted accumulation and dephosphorylation of Bim. These results suggest that BAFF inhibits BCR-induced death by down-regulating Bim via sustained ERK activation, demonstrating that BAFF directly regulates Bim function. Although transitional immature type 1 (T1) B cell numbers are normal in Bim(-/-) mice, T2 and follicular mature B cells are elevated and marginal zone B cells are reduced. Our results suggest that mature B cell homeostasis is maintained by BAFF-mediated regulation of Bim.
Figures
Figure 1.
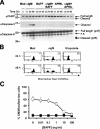
BAFF and APRIL block BCR-induced apoptosis in WEHI-231 B Cells. (A) WEHI-231 cells were treated for 48 h with anti-IgM in the presence or absence of BAFF or APRIL, and apoptotic cells were quantified by FACS using TdT-mediated dUTP nick-end labeling. Results show the means ± SD from three independent experiments. (B) Binding of BAFF to Daudi (left) and WEHI-231 (right) B cell lines was detected by FACS using biotinylated BAFF and streptavidin–PE. Biotinylated BAFF + streptavidin–PE (shaded) and streptavidin–PE only (bold line) traces are shown. Results shown are from one of three experiments. (C) Daudi cells were treated for 48 h with anti-IgM in the presence (open bar) or absence (shaded bar) of BAFF or etoposide (as a positive control), and cell death was quantified by FACS using Mitotracker Red CMXRos. Results shown are from one of two experiments. (D) BAFF-R, TACI, BCMA, and β-actin (as a loading control) were amplified by RT-PCR using gene-specific primers from fourfold serial dilutions of WEHI-231, A20, and splenic B cell cDNA (Table S1, available at
http://www.jem.org/cgi/content/full/jem.20051283/DC1
). PCR products were resolved on NuSieve 3:1 agarose.
Figure 2.
BAFF and APRIL block BCR-induced cell death upstream of caspase-3 and -9 activation and mitochondrial inner membrane depolarization. (A) WEHI-231 cells were treated for the indicated times with anti-IgM antibodies in the presence or absence of BAFF or APRIL. Immunoblots were probed with anti-PARP, anti–cleaved caspase-3, or anti–caspase-9. n.s., nonspecific. (B) WEHI-231 cells were treated for 24 or 48 h with either medium, anti-IgM, or etoposide (as a positive control). (top right) Percentages of Mitotracker Red CMXRos–low cells are shown. (C) WEHI-231 cells were stimulated for 48 h with the indicated concentrations of BAFF in the presence (□) or absence (▪) of anti-IgM. Δψm was determined by FACS using Mitotracker Red CMXRos. Results shown are the means ± SD from three experiments.
Figure 3.
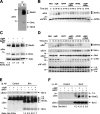
BAFF- and APRIL-induced down-regulation of Bim correlates with a blockade of BCR-induced apoptosis. (A) NP-40 lysates from WEHI-231 cells were immunoprecipitated with anti-Bim mAb or IgG2a isotype control. Immune complexes were probed with anti-Bim serum. Positions of Bim isoforms including multiple BimEL species are shown by arrows. (B) WEHI-231 cells were incubated for 0–48 h with anti-IgM, BAFF, or APRIL (as in Fig. 2 A). Western blots were probed with anti-Bim or anti-APRIL serum (loading control). (C) Purified mouse splenic B cells were incubated with anti-IgM and/or BAFF for 24 h. Immunoblots were probed with anti-Bim or anti-actin serum (loading control). The ratio of BimEL to actin was assessed by scanning densitometry. Dividing lines separate images from different parts of the same gel. (D) WEHI-231 cells were treated for the indicated times with anti-IgM, BAFF, or anti-CD40. Immunoblots were probed with anti-Bim, anti–Bcl-xL, anti-Bmf, or anti–NF-κB2 serum. Nonspecific (n.s.) proteins serve as loading controls. (E and F) WEHI-231 cells were incubated with anti-IgM antibodies and/or BAFF as indicated. Bim (E) or Bcl-2 (F) were immunoprecipitated from NP-40 lysates, which were probed with anti–Bcl-2 or anti-Bim, stripped, and reprobed with anti-Bim or anti–Bcl-2, respectively. Nonspecific (n.s.) bands are indicated. The ratio of Bcl-2 to Bim was quantified by scanning densitometry.
Figure 4.
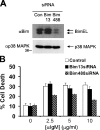
Down-regulation of Bim inhibits BCR-induced apoptosis in WEHI-231 B cells. (A) Immunoblots were prepared as in Fig. 2 and probed with anti-Bim or anti-p38 MAPK (loading control). BimEL species migrating with different mobilities are indicated by arrows. (B) Stable WEHI-231 cell lines expressing either Bim-specific siRNAs or a control vector were treated for 48 h with the indicated doses of anti-IgM, and Δψm was determined by FACS using Mitotracker Red CMXRos. Results shown are the means ± SD from three independent experiments.
Figure 5.
BAFF induces Bim phosphorylation via sustained activation of the MEK–ERK pathway. (A) WEHI-231 cells were treated for 24 h with medium, anti-IgM, BAFF, or anti-CD40. Cell lysates were incubated with or without λ protein phosphatase. Immunoblots were probed with anti-Bim serum. (B) WEHI-231 cells were pretreated for 2 h with DMSO vehicle, 5 μM MEK inhibitor U0126 (U), or 5 μM p38 MAPK inhibitor SB203580 (SB) before incubation with BAFF for 24 h. Blots were probed with anti-Bim, anti–phospho-ERK, or anti-actin (loading control). (C and D) WEHI-231 cells were treated for 0–24 h with anti-IgM in the presence or absence of BAFF. Immunoblots were probed with either anti–phospho-ERK, anti–phospho-MEK, anti–phospho–c-Raf (Ser 338), anti-MKP2, anti-Bim, or anti–Bcl-2 serum. In (D), immunoblots for ERK2 (top) or MEK1/2 (bottom) were quantified by scanning densitometry to measure the fold change in each phosphorylated protein.
Figure 6.
Blockade of BCR-induced apoptosis by chimeric BAFF receptors correlates with changes in Bim mobility and expression. (A) Chimeric BAFF receptors were generated by in-frame fusion of the extracellular and transmembrane domains of mouse CD8α to the cytoplasmic regions of BAFF-R, TACI, and BCMA. (B) Surface levels of chimeric BAFF receptors on clonal WEHI-231 cell lines were quantified by FACS using biotinylated anti-CD8α mAb. Isotype control (open trace) and CD8α cell surface (shaded trace) expression levels are shown. (C and D) Clonal WEHI-231 cell lines expressing chimeric BAFF receptors were incubated for 48 h with the indicated stimuli. Cell death was quantified by trypan blue staining. Results shown are the means ± SD from at least three independent clonal lines. Bim and NF-κB2 were detected by immunoblotting of cell lysates as described in Fig. 3. A dividing line separates images from different parts of the same gel. n.s., nonspecific.
Figure 7.
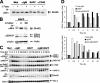
Splenic B cell subsets are dysregulated in Bim KO mice and differ in their expression of BH3-only Bcl-2 family members. (A) Splenic B cells were isolated from WT and Bim−/− mice, stained with αIgD–FITC, αCD21–PE, and αIgM-PerCP, and the numbers of T1, T2-FOP, FO, T2-MZP, and MZ B cells were determined. Data shown are the means ± SEM from three mice per phenotype. MZ B cell numbers in WT and Bim KO mice were 1.7 ± 0.2 × 106 and 0.73 ± 0.09 × 106, respectively. P < 0.05 (*) and P < 0.02 (**) were determined using an unpaired t test. (B) Cell lysates of isolated splenic B cells from WT and Bim−/− mice were probed with anti-Bim or anti-actin (loading control). (C) T1, T2, FO, and MZ splenic B cell subsets were purified by FACS cell sorting using αCD21–FITC, αCD23-APC, and αCD24–PE. Spontaneous cell death was quantified at the indicated times by FACS using Mitotracker Red CMXRos. (D) T1, T2, and FO splenic B cell subsets were isolated as described in C and treated for 18 h with the indicated doses of anti-IgM, and cell death was analyzed as described in C. (E) T1, T2, FO, and MZ splenic B cell subsets were purified as described in C. Bim, Bik, Bmf, Noxa, Puma, and β-actin (loading control) were amplified by RT-PCR (Table S1), and PCR products were resolved as in Fig. 1D. (F) Splenic B cell subsets were isolated by FACS cell sorting as described above. Immunoblots were probed with anti-Bim or anti-actin (loading control).
Similar articles
- MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation.
O'Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, Strasser A. O'Reilly LA, et al. J Immunol. 2009 Jul 1;183(1):261-9. doi: 10.4049/jimmunol.0803853. J Immunol. 2009. PMID: 19542438 Free PMC article. - ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo.
Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. Sabbagh L, et al. J Immunol. 2008 Jun 15;180(12):8093-101. doi: 10.4049/jimmunol.180.12.8093. J Immunol. 2008. PMID: 18523273 - Bim regulates BCR-induced entry of B cells into the cell cycle.
Craxton A, Draves KE, Clark EA. Craxton A, et al. Eur J Immunol. 2007 Oct;37(10):2715-22. doi: 10.1002/eji.200737327. Eur J Immunol. 2007. PMID: 17705137 - The BAFF/APRIL system: life beyond B lymphocytes.
Ng LG, Mackay CR, Mackay F. Ng LG, et al. Mol Immunol. 2005 May;42(7):763-72. doi: 10.1016/j.molimm.2004.06.041. Epub 2004 Dec 8. Mol Immunol. 2005. PMID: 15829264 Review. - Apoptosis and autophagy: BIM as a mediator of tumour cell death in response to oncogene-targeted therapeutics.
Gillings AS, Balmanno K, Wiggins CM, Johnson M, Cook SJ. Gillings AS, et al. FEBS J. 2009 Nov;276(21):6050-62. doi: 10.1111/j.1742-4658.2009.07329.x. Epub 2009 Sep 29. FEBS J. 2009. PMID: 19788418 Review.
Cited by
- Sox4 is required for the survival of pro-B cells.
Sun B, Mallampati S, Gong Y, Wang D, Lefebvre V, Sun X. Sun B, et al. J Immunol. 2013 Mar 1;190(5):2080-9. doi: 10.4049/jimmunol.1202736. Epub 2013 Jan 23. J Immunol. 2013. PMID: 23345330 Free PMC article. - Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF.
Frasca D, Riley RL, Blomberg BB. Frasca D, et al. Exp Gerontol. 2007 Mar;42(3):192-203. doi: 10.1016/j.exger.2006.09.003. Epub 2006 Oct 25. Exp Gerontol. 2007. PMID: 17067770 Free PMC article. - The adaptor molecule Act1 regulates BAFF responsiveness and self-reactive B cell selection during transitional B cell maturation.
Giltiay NV, Lu Y, Allman D, Jørgensen TN, Li X. Giltiay NV, et al. J Immunol. 2010 Jul 1;185(1):99-109. doi: 10.4049/jimmunol.0903312. Epub 2010 Jun 11. J Immunol. 2010. PMID: 20543113 Free PMC article. - TGFβ activated kinase 1 (TAK1) at the crossroad of B cell receptor and Toll-like receptor 9 signaling pathways in human B cells.
Szili D, Bankó Z, Tóth EA, Nagy G, Rojkovich B, Gáti T, Simon M, Hérincs Z, Sármay G. Szili D, et al. PLoS One. 2014 May 6;9(5):e96381. doi: 10.1371/journal.pone.0096381. eCollection 2014. PLoS One. 2014. PMID: 24801688 Free PMC article. - Correlations of TNF-α gene promoter polymorphisms with the risk of thymoma-associated myasthenia gravis in a northern Chinese Han population.
Yang HW, Lei P, Xie YC, Han ZL, Li D, Wang SH, Sun ZL. Yang HW, et al. Cancer Gene Ther. 2017 Jun;24(6):259-266. doi: 10.1038/cgt.2017.13. Epub 2017 Apr 21. Cancer Gene Ther. 2017. PMID: 28429750
References
- Marsden, V.S., and A. Strasser. 2003. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21:71–105. - PubMed
- Strasser, A. 2005. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5:189–200. - PubMed
- Chung, J.B., M. Silverman, and J.G. Monroe. 2003. Transitional B cells: step by step towards immune competence. Trends Immunol. 24:343–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM037905/GM/NIGMS NIH HHS/United States
- GM67905/GM/NIGMS NIH HHS/United States
- P51 RR000166/RR/NCRR NIH HHS/United States
- R01 AI045088/AI/NIAID NIH HHS/United States
- RR00166/RR/NCRR NIH HHS/United States
- AI45088/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous