Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents - PubMed (original) (raw)
Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents
Michal T Krauze et al. J Neurosurg. 2005 Nov.
Abstract
Object: Clinical application of the convection-enhanced delivery (CED) technique is currently limited by low infusion speed and reflux of the delivered agent. The authors developed and evaluated a new step-design cannula to overcome present limitations and to introduce a rapid, reflux-free CED method for future clinical trials.
Methods: The CED of 0.4% trypan blue dye was performed in agarose gel to test cannula needles for distribution and reflux. Infusion rates ranging from 0.5 to 50 microl/minute were used. Agarose gel findings were translated into a study in rats and then in cynomolgus monkeys (Macacafascicularis) by using trypan blue and liposomes to confirm the efficacy of the reflux-free step-design cannula in vivo. Results of agarose gel studies showed reflux-free infusion with high flow rates using the step-design cannula. Data from the study in rats confirmed the agarose gel findings and also revealed increasing tissue damage at a flow rate above 5-microl/minute. Robust reflux-free delivery and distribution of liposomes was achieved using the step-design cannula in brains in both rats and nonhuman primates.
Conclusions: The authors developed a new step-design cannula for CED that effectively prevents reflux in vivo and maximizes the distribution of agents delivered in the brain. Data in the present study show reflux-free infusion with a constant volume of distribution in the rat brain over a broad range of flow rates. Reflux-free delivery of liposomes into nonhuman primate brain was also established using the cannula. This step-design cannula may allow reflux-free distribution and shorten the duration of infusion in future clinical applications of CED in humans.
Figures
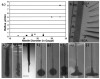
Fig. 1
a: Graph depicting the flow rate (microliter per minute) of reflux for each catheter needle diameter used (18-32 gauge) in agarose gel for the delivery of trypan blue. b: Photograph illustrating the millimeter scale and catheter needles used in Fig. 1a. GA = gauge. c: A 22-gauge catheter needle allowing reflux at a 0.8-μl/minute flow rate. d: Fused silica tubing allowing reflux at a 5-μl/minute flow rate. e: The step-design cannula with fused silica tubing inside cut 1 mm from the cannula tip (12.5 × 1-mm scale, no infusion performed). f: A step-design cannula allowing a 0.5-μl/minute flow rate and 10-μl delivery volume. g: A step-design cannula permitting a 5-μl/minute flow rate and 10-μl delivery volume. h: A step-design cannula allowing a 10-μl/minute flow rate and 10-μl delivery volume. i: Step-design cannula allowing a 20-μl/minute flow rate and 10-μl delivery volume. j: Step-design cannula permitting a 50-μl/minute flow rate and 10-μl delivery volume.
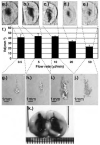
Fig. 2
Histological sections of rodent brain after delivery of 10 μl trypan blue at the following flow rates: 0.5 μl/minute (a), 5 μl/minute (b), 10 μl/minute (c), 20 μl/minute (d), and 50 μl/minute (e). f: Bar graph showing constant Vd from 0.5- to 10-μl/minute flow rate and decreasing Vd at 20 and 50 μl/minute (four cycles for each flow rate). g–j: Tissue damage revealed by H & E staining at the silica tip for the 0.5-, 5-, 10-, and 50-μl/minute flow rates. k: Tissue reflux after 20-μl trypan blue infusion on the 27-gauge catheter side (right) compared with no reflux seen on the step-design cannula side (left).
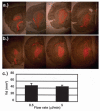
Fig. 3
Photomicrographs of tissue sections exhibiting delivery of 10 μl DiIC18-liposomes into the rat striatum at flow rates of 0.5 μl/minute (a) and 5 μl/minute (b). c: Bar graph depicting the Vd for 10 μl DiIC18-liposomes at 0.5-μl/minute (five) and 5-μl/minute (five) flow rates. Regions generating fluorescence were delineated, and those areas were estimated by using an imaging analysis system.
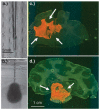
Fig. 4
a: Photograph illustrating the step-design cannula used in the monkey study. b: Image depicting the distribution of 100 μl trypan blue in agarose gel at a 5-μl/minute flow rate. c: Tissue section demonstrating the delivery of 700 μl rhodamine-labeled liposomes into primate corona radiata (arrows). d: Tissue section revealing delivery of 700 μl rhodamine-labeled liposomes into primate brainstem (arrows).
Similar articles
- Optimized cannula design and placement for convection-enhanced delivery in rat striatum.
Yin D, Forsayeth J, Bankiewicz KS. Yin D, et al. J Neurosci Methods. 2010 Mar 15;187(1):46-51. doi: 10.1016/j.jneumeth.2009.12.008. Epub 2009 Dec 22. J Neurosci Methods. 2010. PMID: 20026357 Free PMC article. - Ultrasound-assisted convection-enhanced delivery to the brain in vivo with a novel transducer cannula assembly: laboratory investigation.
Lewis GK Jr, Schulz ZR, Pannullo SC, Southard TL, Olbricht WL. Lewis GK Jr, et al. J Neurosurg. 2012 Dec;117(6):1128-40. doi: 10.3171/2012.7.JNS11144. Epub 2012 Sep 21. J Neurosurg. 2012. PMID: 22998056 - Intraparenchymal ultrasound application and improved distribution of infusate with convection-enhanced delivery in rodent and nonhuman primate brain.
Mano Y, Saito R, Haga Y, Matsunaga T, Zhang R, Chonan M, Haryu S, Shoji T, Sato A, Sonoda Y, Tsuruoka N, Nishiyachi K, Sumiyoshi A, Nonaka H, Kawashima R, Tominaga T. Mano Y, et al. J Neurosurg. 2016 May;124(5):1490-500. doi: 10.3171/2015.3.JNS142152. Epub 2015 Oct 23. J Neurosurg. 2016. PMID: 26495939 - A realistic brain tissue phantom for intraparenchymal infusion studies.
Chen ZJ, Gillies GT, Broaddus WC, Prabhu SS, Fillmore H, Mitchell RM, Corwin FD, Fatouros PP. Chen ZJ, et al. J Neurosurg. 2004 Aug;101(2):314-22. doi: 10.3171/jns.2004.101.2.0314. J Neurosurg. 2004. PMID: 15309925 Review. - Convection-enhanced delivery in the treatment of malignant glioma.
Lopez KA, Waziri AE, Canoll PD, Bruce JN. Lopez KA, et al. Neurol Res. 2006 Jul;28(5):542-8. doi: 10.1179/016164106X116836. Neurol Res. 2006. PMID: 16808887 Review.
Cited by
- Controlled Catheter Movement Affects Dye Dispersal Volume in Agarose Gel Brain Phantoms.
Mehta JN, McRoberts GR, Rylander CG. Mehta JN, et al. Pharmaceutics. 2020 Aug 11;12(8):753. doi: 10.3390/pharmaceutics12080753. Pharmaceutics. 2020. PMID: 32796527 Free PMC article. - Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts.
Krauze MT, Noble CO, Kawaguchi T, Drummond D, Kirpotin DB, Yamashita Y, Kullberg E, Forsayeth J, Park JW, Bankiewicz KS. Krauze MT, et al. Neuro Oncol. 2007 Oct;9(4):393-403. doi: 10.1215/15228517-2007-019. Epub 2007 Jul 24. Neuro Oncol. 2007. PMID: 17652269 Free PMC article. - Convection-Enhanced Arborizing Catheter System Improves Local/Regional Delivery of Infusates Versus a Single-Port Catheter in Ex Vivo Porcine Brain Tissue.
Elenes EY, Mehta JN, Hsu FC, Whitlow CT, Debinski W, Rossmeisl J, Tatter S, Rylander CG. Elenes EY, et al. J Eng Sci Med Diagn Ther. 2021 Feb 1;4(1):011003. doi: 10.1115/1.4048935. Epub 2020 Dec 2. J Eng Sci Med Diagn Ther. 2021. PMID: 35832263 Free PMC article. - Safety of real-time convection-enhanced delivery of liposomes to primate brain: a long-term retrospective.
Krauze MT, Vandenberg SR, Yamashita Y, Saito R, Forsayeth J, Noble C, Park J, Bankiewicz KS. Krauze MT, et al. Exp Neurol. 2008 Apr;210(2):638-44. doi: 10.1016/j.expneurol.2007.12.015. Epub 2007 Dec 27. Exp Neurol. 2008. PMID: 18295759 Free PMC article. - Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma.
Sperring CP, Argenziano MG, Savage WM, Teasley DE, Upadhyayula PS, Winans NJ, Canoll P, Bruce JN. Sperring CP, et al. Neurooncol Adv. 2023 Apr 21;5(1):vdad044. doi: 10.1093/noajnl/vdad044. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37215957 Free PMC article. Review.
References
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. - PubMed
- Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, et al. Intracerebral clysis in a rat glioma model. Neurosurgery. 2000;46:683–691. - PubMed
- Chen ZJ, Broaddus WC, Viswanathan RR, Raghavan R, Gillies GT. Intraparenchymal drug delivery via positive-pressure infusion: experimental and modeling studies of poroelasticity in brain phantom gels. IEEE Trans Biomed Eng. 2002;49:85–96. - PubMed
- Chen ZJ, Gillies GT, Broaddus WC, Prabhu SS, Fillmore H, Mitchell RM, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. J Neurosurg. 2004;101:314–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA097257/CA/NCI NIH HHS/United States
- U54 NS045309/NS/NINDS NIH HHS/United States
- U54 NS045309-010001/NS/NINDS NIH HHS/United States
- P50 CA097257-01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources