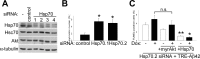Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response - PubMed (original) (raw)
Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response
Jordi Magrané et al. J Neurosci. 2005.
Abstract
Early events in Alzheimer's disease (AD) pathogenesis implicate the accumulation of beta-amyloid (Abeta) peptide inside neurons in vulnerable brain regions. However, little is known about the consequences of intraneuronal Abeta on signaling mechanisms. Here, we demonstrate, using an inducible viral vector system to drive intracellular expression of Abeta42 peptide in primary neuronal cultures, that this accumulation results in the inhibition of the Akt survival signaling pathway. Induction of intraneuronal Abeta42 expression leads to a sequential decrease in levels of phospho-Akt, increase in activation of glycogen synthase kinase-3beta, and apoptosis. Downregulation of Akt also paralleled intracellular Abeta accumulation in vivo in the Tg2576 AD mouse model. Overexpression of constitutively active Akt reversed the toxic effects of Abeta through a mechanism involving the induction of heat shock proteins (Hsps). We used a small-interfering RNA approach to explore the possibility of a link between Akt activity and Hsp70 expression and concluded that neuroprotection by Akt could be mediated through downstream induction of Hsp70 expression. These results suggest that the early dysfunction associated with intraneuronal Abeta accumulation in AD involve the associated impairments of Akt signaling and suppression of the stress response.
Figures
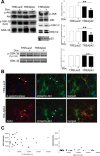
Figure 1.
Intracellularly expressed Aβ42 downregulates the Akt survival pathway. Primary rat cortical neurons were infected with AdTet-On and either AdTRE-LacZ or AdTRE-Aβ42 as indicated, and transgene expression was induced by 1 μg/ml Dox for 24 h (+, top). A, Phospho-protein levels of Akt, GSK-3 β (Ser9 and Tyr216), members of the MAPK pathway, and levels of p53 were analyzed by Western blot. Corresponding total protein levels showed equivalent protein loading. Densitometric quantitation expressed as the relative phospho-protein to total protein ratio (n = 3; *p < 0.005; **p < 0.05; n.s., nonsignificant). Error bars represent 1 SE. B, Immunofluorescent images from primary neurons infected with AdTet-On and AdTRE-LacZ (top) or AdTRE-Aβ42 (bottom). Cells were immunostained for transgene expression (red) and phospho-Akt levels (green); merged images are on the right. C, Quantification of phospho-Akt fluorescence relative to transgene expression. Closed circles, Phospho-Akt intensity in either Aβ42 or β-galactosidase (β-gal)-expressing neurons; open circles, phospho-Akt intensity in noninfected cells.
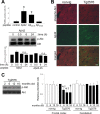
Figure 2.
Effects of extracellularly applied Aβ peptide in vitro and intraneuronal accumulation of Aβ42 in vivo. A, Extracellularly applied Aβ peptides result in apoptosis through a mechanism independent of the Akt pathway. Different Aβ peptides were added to the culture medium at a final concentration of 10 μ
m
for 24 h. Quantification of TUNEL staining data from four independent experiments is shown (comparison of treated cultures vs control; *p < 0.0005). Levels of phospho-Akt after Aβ42 peptide treatment over time. Densitometric quantitation expressed as the relative phospho-Akt to total Akt ratio for Aβ42 or Aβ25-35 peptide-treated cultures. n.s., Nonsignificant compared with untreated controls. B, Cortical sections of nontransgenic (non-tg) and Tg2576 mice were analyzed for intraneuronally accumulated Aβ42 and phospho-Akt by confocal microscopy. Scale bar, 20 μm. C, Phospho-Akt levels decreased with age in frontal cortex extracts from Tg2576 mice. Equal amounts of proteins were loaded. Quantitative data on relative changes of phospho-Akt levels in the frontal cortex and cerebellum of nontransgenic (non-tg) and Tg2576 mice (n = 3; *p < 0.05, relative to 2 months; †p < 0.05, relative to its companion age of nontransgenic). Error bars represent 1 SE.
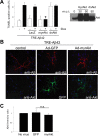
Figure 3.
Akt overexpression rescues Aβ42-induced toxicity without affecting levels of intraneuronal Aβ. A, Cortical neurons were infected with AdTet-On and AdTRE-Aβ42. Transgene expression was induced by 1 μg/ml Dox for 24 h (+, bottom), and cells were coinfected with Ad-LacZ, Ad-myrAkt (constitutively active Akt), or Ad-dnAkt (inactive Akt) as indicated. Ad-myrAkt fully protected against Aβ42-induced neuronal death, as measured by TUNEL staining (*p < 0.005). Coinfection with Ad-dnAkt or Ad-LacZ control did not affect Aβ42 toxicity. Values from three independent experiments are shown. Immunoblot analysis of Akt overexpression in primary neurons (right). B, Immunofluorescent images from primary neurons infected with AdTet-On and AdTRE-Aβ42. Cells were coinfected with Ad-GFP or Ad-myrAkt, as indicated. No apparent changes in levels or localization of Aβ were observed. C, Quantification of Aβ immunofluorescence from neurons with or without coexpression of GFP control or myrAkt. Ratio of Aβ42 intensity in processes divided by that in cell bodies was calculated, with the ratio standardized to 1.0 for cells without GFP or myrAkt coexpression. Error bars represent 1 SE. Results from three independent experiments are shown. n.s., Nonsignificant.
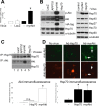
Figure 4.
Akt overexpression induces the neuronal stress response. A, Luciferase assay performed on neuroblastoma cells first infected with Ad-HSP-Luc reporter and then with the indicated viruses (see Materials and Methods). MyrAkt overexpression, but not controls, activated the Hsp70B promoter and induced luciferase activity. Values are presented as x-fold increase compared with control (n = 5; p < 0.0005). B, Immunoblot of proteins extracted from primary neuronal cultures that were uninfected or infected with different viral vectors, as indicated. Equal amounts of protein were loaded. Overexpression of Akt (HA-tagged), but not of other transgenes, induced Hsp70 expression (left). Analysis of the induction of the stress response in neuroblastoma cells (right). C, Neuroblastoma cells were infected with viruses (top), and cell extracts were immunoprecipitated with anti-Hsp70 or anti-Akt antibodies and probed for the indicated proteins. Akt overexpression increased the amount of Hsp70 available for immunoprecipitation from extracts (top, lane 4), but Hsp70 overexpression had no effect on Akt levels (middle, lane 5). Increased recovery of Hsp70 did not occur in Akt-Hsp70 coimmunoprecipitations (bottom, lane 4). D, Immunofluorescent images from primary neurons infected with no virus, Ad-Hsp70, or Ad-myrAkt and immunostained for total Akt (green) and Hsp70 (red). Akt overexpression in neurons correlated with increased Hsp70 immunostaining, whereas Hsp70 had no effects on Akt levels. Quantification of fluorescence for Akt and Hsp70 expression from several independent experiments is shown. Error bars represent 1 SE. n.s., Nonsignificant. *p < 0.01.
Figure 5.
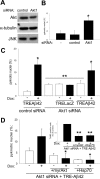
Akt and Hsp70 overexpression can reverse Aβ42-induced cell death in Akt1-depleted neurons. A, Primary neurons were treated for 72 h with rhodamine-control or Akt1 siRNA, and cell lysates were analyzed using the indicated antibodies. Total Akt1 levels were reduced ∼20%, whereas JNK and α-tubulin levels were unaffected (representative blots are shown). B, Suppression of endogenous Akt1 protein in primary neurons produced a threefold increase in neuronal death under basal conditions, as measured by nuclear pyknosis (n = 3; *p < 0.005). C, Primary neurons were treated with rhodamine-control or Akt1 siRNA and infected with either AdTRE-LacZ or AdTRE-Aβ42 viruses. Induction of transgene expression with Dox (+) increased cell death in Aβ42-expressing cells (n = 3; *p < 0.05, compared with noninduced cultures) but not in β-galactosidase-expressing controls. Suppression of Akt1 caused an overall increase in neuronal death (**p < 0.05, compared with noninduced control siRNA). D, Primary neurons and neuroblastoma cells (inset) were treated with Akt1 siRNA and infected with AdTRE-Aβ42 viruses. Transgene expression was then induced with Dox (+), and cells were infected with either Ad-myrAkt or Ad-Hsp70, as indicated (see Materials and Methods for details). Apoptotic death increased in Akt1-depleted Aβ42-expressing cultures. Coinfection with either Ad-myrAkt or Ad-Hsp70 reversed the observed toxic effects of Aβ42 expression in both cell types (n = 3; *p < 0.05 and **p < 0.005, compared with Akt1-depleted Aβ42-expressing cells). Error bars represent 1 SE.
Figure 6.
Hsp70 acts downstream of Akt. A, Immunoblot analysis of primary neuronal cultures treated with no siRNA (-), rhodamine-control siRNA, or different Hsp70 siRNA (1-4). Hsp70 protein levels were reduced 30-70%; however, levels of noninducible Hsc70, Akt, and α-tubulin were not affected. B, Depletion of endogenous Hsp70 (siRNA Hsp70.1 and Hsp70.2) from cortical neurons induced cell death, as measured by nuclear pyknosis (n = 3; *p < 0.05). C, Neuroblastoma cells were treated with Hsp70.2 siRNA, infected with AdTet-On and AdTRE-Aβ42 viruses, and transgene expression induced with Dox (+). Overexpression of Hsp70 was shown to reverse TUNEL labeling caused by Hsp70 depletion alone and in combination with Aβ42 accumulation (*p < 0.0005, compared with Aβ42-expressing cultures; **p < 0.05, compared with noninduced cultures). Coinfection with Ad-myrAkt had a nonstatistically significant effect. Error bars represent 1 SE. Values from four independent experiments are shown.
Similar articles
- Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons.
Magrané J, Smith RC, Walsh K, Querfurth HW. Magrané J, et al. J Neurosci. 2004 Feb 18;24(7):1700-6. doi: 10.1523/JNEUROSCI.4330-03.2004. J Neurosci. 2004. PMID: 14973234 Free PMC article. - mTORC2 (Rictor) in Alzheimer's Disease and Reversal of Amyloid-β Expression-Induced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons.
Lee HK, Kwon B, Lemere CA, de la Monte S, Itamura K, Ha AY, Querfurth HW. Lee HK, et al. J Alzheimers Dis. 2017;56(3):1015-1036. doi: 10.3233/JAD-161029. J Alzheimers Dis. 2017. PMID: 28035937 Free PMC article. - Beta-amyloid1-42 gene transfer model exhibits intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss.
Rebeck GW, Hoe HS, Moussa CE. Rebeck GW, et al. J Biol Chem. 2010 Mar 5;285(10):7440-6. doi: 10.1074/jbc.M109.083915. Epub 2010 Jan 13. J Biol Chem. 2010. PMID: 20071340 Free PMC article. - Melatonin protects against Aβ-induced neurotoxicity in primary neurons via miR-132/PTEN/AKT/FOXO3a pathway.
Zhao Y, Zhao R, Wu J, Wang Q, Pang K, Shi Q, Gao Q, Hu Y, Dong X, Zhang J, Sun J. Zhao Y, et al. Biofactors. 2018 Nov;44(6):609-618. doi: 10.1002/biof.1411. Epub 2018 Jan 11. Biofactors. 2018. PMID: 29322615 - Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms.
Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Collins MA, et al. Mol Neurobiol. 2010 Jun;41(2-3):420-5. doi: 10.1007/s12035-010-8138-0. Epub 2010 Apr 28. Mol Neurobiol. 2010. PMID: 20422315 Free PMC article. Review.
Cited by
- The polarity protein Par3 regulates APP trafficking and processing through the endocytic adaptor protein Numb.
Sun M, Asghar SZ, Zhang H. Sun M, et al. Neurobiol Dis. 2016 Sep;93:1-11. doi: 10.1016/j.nbd.2016.03.022. Epub 2016 Apr 9. Neurobiol Dis. 2016. PMID: 27072891 Free PMC article. - Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics.
Jope RS, Yuskaitis CJ, Beurel E. Jope RS, et al. Neurochem Res. 2007 Apr-May;32(4-5):577-95. doi: 10.1007/s11064-006-9128-5. Epub 2006 Aug 30. Neurochem Res. 2007. PMID: 16944320 Free PMC article. Review. - Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease.
Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Sofola O, et al. PLoS Genet. 2010 Sep 2;6(9):e1001087. doi: 10.1371/journal.pgen.1001087. PLoS Genet. 2010. PMID: 20824130 Free PMC article. - Synaptic basis of Alzheimer's disease: Focus on synaptic amyloid beta, P-tau and mitochondria.
John A, Reddy PH. John A, et al. Ageing Res Rev. 2021 Jan;65:101208. doi: 10.1016/j.arr.2020.101208. Epub 2020 Nov 4. Ageing Res Rev. 2021. PMID: 33157321 Free PMC article. Review. - ICP10PK inhibits calpain-dependent release of apoptosis-inducing factor and programmed cell death in response to the toxin MPP+.
Wales SQ, Laing JM, Chen L, Aurelian L. Wales SQ, et al. Gene Ther. 2008 Oct;15(20):1397-409. doi: 10.1038/gt.2008.88. Epub 2008 May 22. Gene Ther. 2008. PMID: 18496573 Free PMC article.
References
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC (2004) Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res 297: 186-196. - PubMed
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295: 865-868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS41371/NS/NINDS NIH HHS/United States
- AG17241/AG/NIA NIH HHS/United States
- R01 AG017241/AG/NIA NIH HHS/United States
- AG009464/AG/NIA NIH HHS/United States
- P01 AG009464/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources