Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2 - PubMed (original) (raw)
Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2
Dennis Mathew et al. Science. 2005.
Abstract
Wingless secretion provides pivotal signals during development by activating transcription of target genes. At Drosophila synapses, Wingless is secreted from presynaptic terminals and is required for synaptic growth and differentiation. Wingless binds the seven-pass transmembrane DFrizzled2 receptor, but the ensuing events at synapses are not known. We show that DFrizzled2 is endocytosed from the postsynaptic membrane and transported to the nucleus. The C terminus of DFrizzled2 is cleaved and translocated into the nucleus; the N-terminal region remains just outside the nucleus. Translocation of DFrizzled2-C into the nucleus, but not its cleavage and transport, depends on Wingless signaling. We conclude that, at synapses, Wingless signal transduction occurs through the nuclear localization of DFrizzled2-C for potential transcriptional regulation of synapse development.
Figures
Fig. 1
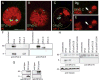
Localization of DFz2-C and DFz2-N to the same NMJs, but to different subcellular compartments, inside the nucleus (DFz2-C) and at the perinuclear region (DFz2-N). (A and B) Wild-type third instar NMJs from muscles 6 and 7 (A3) stained with antibodies against (A) DFz2-C and (B) DFz2-N. (C through J) Representative muscle nuclei at muscle 6 (A3) in preparations double-stained with antibodies against (C through F) DFz2-C (green) and tubulin (red) and (G through J) DFz-N (blue) and tubulin (Tub) (red). Note that (C to F) and (G to J) were obtained from different preparations. (C, D, G, H) show a confocal slice at a focal plane through the nuclear-cytoplasmic boundary (defined by the microtubular array; tangential), and (E, F, I, J) a confocal slice at a focal plane midway through the nucleus (medial). N, nucleus, arrowheads in C point to cytoplasmic DFz2-C. Scale bar, 9 μm in (A and B), and 8 μm in (C to J).
Fig. 2
Localization of DFz2-C to euchromatin and evidence for cleavage of DFz2. (A and B) Colocalization of (A) DFz2-C (green) and PI (red) and (B) DFz2-C and OSA. In (C to E), muscle nuclei were double-labeled with antibodies against the heterochromatin-specific protein HP-1 (red) and DFz2-C (green). Arrows point to regions of adjacent DFz2-C and HP-1 immunoreactivity. Scale bar, 9 μm in (A to C), and 6 μm in (D and E). (F) Western blot of S2 cells transfected with full-length DFz2, DFz2-N, and DFz2-C. (G) Western blots of body wall muscle extracts from wild-type larvae and larvae overexpressing full-length DFz2. (H) Western blot of S2 cells and S2 cells transfected with DFz2 constructs. Blots were sequentially probed with antibodies to DFz2-C, DFz2-N, and tubulin.
Fig. 3
In vivo transport of DFz2 from the cell surface to the nucleus. (A to F) show anti-DFz2-N immunoreactivity at NMJs during the in vivo DFz2 internalization assay. (A and D) Surface DFz2 (blue) and (B and E) internalized DFz2 (magenta) are shown at 5 and 60 min after pulse-labeling. (C and F) show the merged blue and magenta channels. (G and H) DFz2-N immunoreactivity around a muscle nucleus at 5 and 60 min after pulse-labeling. Scale bar, 9 μm in (A to F), and 6 μm in (G and H).
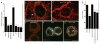
Fig. 4
Role of endocytosis, retrograde transport, and Wg signaling in DFz2-C nuclear import. (A) Mean number of nuclear spots per nucleus in different genotypes. (B and C) Muscle nuclei in larva expressing (B) DFz2 and (C) DFz2 and Shi-DN in the muscles. Tissues were double-labeled with antibodies against tubulin and DFz2-C. Arrow in (B) points to DFz2-C spots that accumulate just outside of the nuclear perimeter. (D) Number of synaptic boutons in wild type and dfz2C1/Dfdfz2 mutants expressing various DFz2 transgenes in muscle as indicated. ***P < 0.0001 compared with wild type [ANOVA (20)]. (E to G) DFz2-C and tubulin immunoreactivity in (E) S2 cells, and [(F and G)] S2 transfected with DFz2, and treated with and without Wg-conditioned medium. Scale bar, 9 μm in (B) and (C), and 7 μm in (E) to (G).
Comment in
- Cell signaling. Frizzled at the cutting edge of the synapse.
Martinez Arias A. Martinez Arias A. Science. 2005 Nov 25;310(5752):1284-5. doi: 10.1126/science.1121906. Science. 2005. PMID: 16311322 No abstract available.
Similar articles
- Cell signaling. Frizzled at the cutting edge of the synapse.
Martinez Arias A. Martinez Arias A. Science. 2005 Nov 25;310(5752):1284-5. doi: 10.1126/science.1121906. Science. 2005. PMID: 16311322 No abstract available. - Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development.
Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Bhanot P, et al. Development. 1999 Sep;126(18):4175-86. doi: 10.1242/dev.126.18.4175. Development. 1999. PMID: 10457026 - Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP.
Ataman B, Ashley J, Gorczyca D, Gorczyca M, Mathew D, Wichmann C, Sigrist SJ, Budnik V. Ataman B, et al. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7841-6. doi: 10.1073/pnas.0600387103. Epub 2006 May 8. Proc Natl Acad Sci U S A. 2006. PMID: 16682643 Free PMC article. - Wingless signaling: the inconvenient complexities of life.
Cox RT, Peifer M. Cox RT, et al. Curr Biol. 1998 Feb 12;8(4):R140-4. doi: 10.1016/s0960-9822(98)70081-8. Curr Biol. 1998. PMID: 9501979 Review. No abstract available. - Interactions between wingless and frizzled molecules in Drosophila.
Nusse R, Rulifson E, Fish M, Harryman-Samos C, Brink M, Wu CH, Cadigan K. Nusse R, et al. Ernst Schering Res Found Workshop. 2000;(29):1-11. doi: 10.1007/978-3-662-04264-9_1. Ernst Schering Res Found Workshop. 2000. PMID: 10943301 Review. No abstract available.
Cited by
- Activity-dependent retrograde laminin A signaling regulates synapse growth at Drosophila neuromuscular junctions.
Tsai PI, Wang M, Kao HH, Cheng YJ, Lin YJ, Chen RH, Chien CT. Tsai PI, et al. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17699-704. doi: 10.1073/pnas.1206416109. Epub 2012 Oct 10. Proc Natl Acad Sci U S A. 2012. PMID: 23054837 Free PMC article. - GPR50-Ctail cleavage and nuclear translocation: a new signal transduction mode for G protein-coupled receptors.
Ahmad R, Lahuna O, Sidibe A, Daulat A, Zhang Q, Luka M, Guillaume JL, Gallet S, Guillonneau F, Hamroune J, Polo S, Prévot V, Delagrange P, Dam J, Jockers R. Ahmad R, et al. Cell Mol Life Sci. 2020 Dec;77(24):5189-5205. doi: 10.1007/s00018-019-03440-7. Epub 2020 Jan 3. Cell Mol Life Sci. 2020. PMID: 31900622 Free PMC article. - Glial wingless/Wnt regulates glutamate receptor clustering and synaptic physiology at the Drosophila neuromuscular junction.
Kerr KS, Fuentes-Medel Y, Brewer C, Barria R, Ashley J, Abruzzi KC, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR, Budnik V. Kerr KS, et al. J Neurosci. 2014 Feb 19;34(8):2910-20. doi: 10.1523/JNEUROSCI.3714-13.2014. J Neurosci. 2014. PMID: 24553932 Free PMC article. - Two classes of matrix metalloproteinases reciprocally regulate synaptogenesis.
Dear ML, Dani N, Parkinson W, Zhou S, Broadie K. Dear ML, et al. Development. 2016 Jan 1;143(1):75-87. doi: 10.1242/dev.124461. Epub 2015 Nov 24. Development. 2016. PMID: 26603384 Free PMC article. - Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling.
Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Ataman B, et al. Neuron. 2008 Mar 13;57(5):705-18. doi: 10.1016/j.neuron.2008.01.026. Neuron. 2008. PMID: 18341991 Free PMC article.
References
- Hall AC, Lucas FR, Salinas PC. Cell. 2000;100:525. - PubMed
- Krylova O, et al. Neuron. 2002;35:1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD036000/HD/NICHD NIH HHS/United States
- R01 MH070000/MH/NIMH NIH HHS/United States
- GM R01 HD36000/GM/NIGMS NIH HHS/United States
- R01 MH70000/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases