Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice - PubMed (original) (raw)
Reexamination of the role of ubiquitin-like modifier ISG15 in the phenotype of UBP43-deficient mice
Klaus-Peter Knobeloch et al. Mol Cell Biol. 2005 Dec.
Abstract
UBP43/USP18 was described as a specific protease that removes conjugated ubiquitin-like modifier ISG15 from target proteins. The severe phenotype of UBP43(-/-) mice characterized by premature death, brain cell injury, and deregulated STAT1 signaling was ascribed to an enhanced conjugation of ISG15. In contrast, no phenotypic changes were detected in ISG15(-/-) mice. To verify the role of ISG15 in the phenotype of UBP43(-/-) mice, we employed mice deficient for both ISG15 and UBP43. Here, we show that the phenotype of UBP43(-/-) mice was not rescued by the absence of ISG15, as evident from unchanged mortality, neurological symptoms, and occurrence of hydrocephalus. Also, the reported hypersensitivity of UBP43(-/-) mice to an interferon inducer, poly(I . C), was ISG15 independent. Furthermore, no evidence for a role of ISG15 in the modulation of STAT1 signaling or in the resistance against lymphocytic choriomeningitis virus and vesicular stomatitis virus was found. Presented results clearly demonstrate that the phenotypic alterations of UBP43(-/-) mice are not caused by the lack of ISG15 deconjugation and must be due to another, non-ISG15-mediated molecular mechanism.
Figures
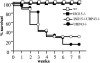
FIG. 1.
Mortality of ISG15−/− UBP43−/− and UBP43−/− mice. Survival of wild-type (n = 25), ISG15−/− (n = 25), UBP43−/− (n = 36), and ISG15−/− UBP43−/− (n = 24) mice was monitored for a time period of 8 weeks. wt, wild type.
FIG. 2.
Hydrocephalus in UBP43−/− and UBP43−/− ISG15−/− mice. Macroscopic images of sculls (A) and hematoxylin-eosin stained coronal sections (B) of brains derived from 8-week-old mice of the indicated genotypes. wt, wild type.
FIG. 3.
Hypersensitivity to poly(I · C). Sensitivity of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− mice to poly(I · C) treatment was assessed by intraperitoneal injection of mice with poly(I · C) (50 mg/g of body weight).
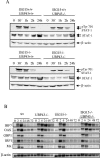
FIG. 4.
STAT1 responses of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− MEF. (A) MEF were stimulated with 100 U/ml of IFN-β for the times indicated. Western blots were incubated with anti-pTyr701 antibody to detect STAT1 phosphorylation and with anti-STAT1 and β-actin as loading controls. (B) MEF were stimulated with 100 U/ml IFN-β for the times indicated. Upon RNA isolation, Northern blots were hybridized with probes specific for the STAT-1 target genes IRF7, 2′,5′ oligoadenylate synthetase (OAS), guanylate-binding protein-1 (GBP1), IRF1, and Mx.1, with β-actin as a loading control. wt, wild type.
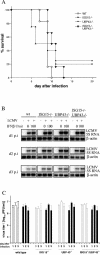
FIG. 5.
Viral infections. (A) Wild-type (n = 6), UBP43−/− (n = 4), ISG15−/− (n = 5), and ISG15−/− UBP43−/− (n = 6) mice were infected intracranially with 105 IU of LCMV, and survival was monitored daily. (B) RNA from MEF of the indicated genotypes pretreated with the indicated amounts of IFN-β was isolated 1, 2, or 3 days postinfection (d1 p.i., d2 p.i., and d3 p.i., respectively) with LCMV, as described in Materials and Methods. RNA was separated, and Northern blots were hybridized using a probe specific for 5S LCMV and for actin as a loading control, respectively. (C) Virus replication in wild-type, UBP43−/−, ISG15−/−, and ISG15−/− UBP43−/− MEF either untreated (open bars) or treated with 100 U/ml IFN-β (filled bars) 24 h prior to infection with LCMV was assessed by measuring viral titers in the supernatant 1, 2, or 3 days after infection as described in Materials and Methods. wt, wild type.
FIG. 6.
Antiviral response of wild-type, UBP43, ISG15−/−, and ISG15−/− UBP43−/− cells upon VSV infection. MEF were incubated with serial dilutions of recombinant murine IFN-β as indicated. After 24 h, cell cultures were infected with VSV and cell viability was monitored 24 h later. wt, wild type.
Similar articles
- ISG15, an interferon-stimulated ubiquitin-like protein, is not essential for STAT1 signaling and responses against vesicular stomatitis and lymphocytic choriomeningitis virus.
Osiak A, Utermöhlen O, Niendorf S, Horak I, Knobeloch KP. Osiak A, et al. Mol Cell Biol. 2005 Aug;25(15):6338-45. doi: 10.1128/MCB.25.15.6338-6345.2005. Mol Cell Biol. 2005. PMID: 16024773 Free PMC article. - Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling.
Kim KI, Yan M, Malakhova O, Luo JK, Shen MF, Zou W, de la Torre JC, Zhang DE. Kim KI, et al. Mol Cell Biol. 2006 Jan;26(2):472-9. doi: 10.1128/MCB.26.2.472-479.2006. Mol Cell Biol. 2006. PMID: 16382139 Free PMC article. - ISG15, not just another ubiquitin-like protein.
Kim KI, Zhang DE. Kim KI, et al. Biochem Biophys Res Commun. 2003 Aug 1;307(3):431-4. doi: 10.1016/s0006-291x(03)01216-6. Biochem Biophys Res Commun. 2003. PMID: 12893238 Review. - Interferon-stimulated gene 15 and the protein ISGylation system.
Zhang D, Zhang DE. Zhang D, et al. J Interferon Cytokine Res. 2011 Jan;31(1):119-30. doi: 10.1089/jir.2010.0110. Epub 2010 Dec 29. J Interferon Cytokine Res. 2011. PMID: 21190487 Free PMC article. Review.
Cited by
- USP18 recruits USP20 to promote innate antiviral response through deubiquitinating STING/MITA.
Zhang M, Zhang MX, Zhang Q, Zhu GF, Yuan L, Zhang DE, Zhu Q, Yao J, Shu HB, Zhong B. Zhang M, et al. Cell Res. 2016 Dec;26(12):1302-1319. doi: 10.1038/cr.2016.125. Epub 2016 Nov 1. Cell Res. 2016. PMID: 27801882 Free PMC article. - Viral defense, carcinogenesis and ISG15: novel roles for an old ISG.
Pitha-Rowe IF, Pitha PM. Pitha-Rowe IF, et al. Cytokine Growth Factor Rev. 2007 Oct-Dec;18(5-6):409-17. doi: 10.1016/j.cytogfr.2007.06.017. Epub 2007 Aug 3. Cytokine Growth Factor Rev. 2007. PMID: 17689132 Free PMC article. Review. - Identification and Validation of ISG15 Target Proteins.
Durfee LA, Huibregtse JM. Durfee LA, et al. Subcell Biochem. 2010;54:228-37. doi: 10.1007/978-1-4419-6676-6_18. Subcell Biochem. 2010. PMID: 21222286 Free PMC article. Review. - Deletion of the deISGylating enzyme USP18 enhances tumour cell antigenicity and radiosensitivity.
Pinto-Fernandez A, Salio M, Partridge T, Chen J, Vere G, Greenwood H, Olie CS, Damianou A, Scott HC, Pegg HJ, Chiarenza A, Díaz-Saez L, Smith P, Gonzalez-Lopez C, Patel B, Anderton E, Jones N, Hammonds TR, Huber K, Muschel R, Borrow P, Cerundolo V, Kessler BM. Pinto-Fernandez A, et al. Br J Cancer. 2021 Feb;124(4):817-830. doi: 10.1038/s41416-020-01167-y. Epub 2020 Nov 20. Br J Cancer. 2021. PMID: 33214684 Free PMC article. - ITAM-coupled receptors inhibit IFNAR signaling and alter macrophage responses to TLR4 and Listeria monocytogenes.
Huynh L, Wang L, Shi C, Park-Min KH, Ivashkiv LB. Huynh L, et al. J Immunol. 2012 Apr 1;188(7):3447-57. doi: 10.4049/jimmunol.1102211. Epub 2012 Feb 24. J Immunol. 2012. PMID: 22368279 Free PMC article.
References
- Farrell, P. J., R. J. Broeze, and P. Lengyel. 1979. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature 279:523-525. - PubMed
- Gegin, C., and F. Lehmann-Grube. 1992. Control of acute infection with lymphocytic choriomeningitis virus in mice that cannot present an immunodominant viral cytotoxic T lymphocyte epitope. J. Immunol. 149:3331-3338. - PubMed
- Haas, A. L., P. Ahrens, P. M. Bright, and H. Ankel. 1987. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J. Biol. Chem. 262:11315-11323. - PubMed
- Hamerman, J. A., F. Hayashi, L. A. Schroeder, S. P. Gygi, A. L. Haas, L. Hampson, P. Coughlin, R. Aebersold, and A. Aderem. 2002. Serpin 2a is induced in activated macrophages and conjugates to a ubiquitin homolog. J. Immunol. 168:2415-2423. - PubMed
- Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous