Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT - PubMed (original) (raw)
Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT
Brad A Bryan et al. Mol Cell Biol. 2005 Dec.
Abstract
Rho family guanine nucleotide exchange factors (GEFs) regulate diverse cellular processes including cytoskeletal reorganization, cell adhesion, and differentiation via activation of the Rho GTPases. However, no studies have yet implicated Rho-GEFs as molecular regulators of the mesenchymal cell fate decisions which occur during development and repair of tissue damage. In this study, we demonstrate that the steady-state protein level of the Rho-specific GEF GEFT is modulated during skeletal muscle regeneration and that gene transfer of GEFT into cardiotoxin-injured mouse tibialis anterior muscle exerts a powerful promotion of skeletal muscle regeneration in vivo. In order to molecularly characterize this regenerative effect, we extrapolate the mechanism of action by examining the consequence of GEFT expression in multipotent cell lines capable of differentiating into a number of cell types, including muscle and adipocyte lineages. Our data demonstrate that endogenous GEFT is transcriptionally upregulated during myogenic differentiation and downregulated during adipogenic differentiation. Exogenous expression of GEFT promotes myogenesis of C2C12 cells via activation of RhoA, Rac1, and Cdc42 and their downstream effector proteins, while a dominant-negative mutant of GEFT inhibits this process. Moreover, we show that GEFT inhibits insulin-induced adipogenesis in 3T3L1 preadipocytes. In summary, we provide the first evidence that the Rho family signaling pathways act as potential regulators of skeletal muscle regeneration and provide the first reported molecular mechanism illustrating how a mammalian Rho family GEF controls this process by modulating mesenchymal cell fate decisions.
Figures
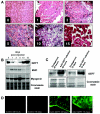
FIG. 1.
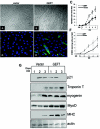
Infection of AAV2/8-GEFT in the mouse tibialis anterior muscle. (A) Representative immunohistochemical sections of cardiotoxin-injured tibialis anterior muscle sections stained with H&E and taken from untreated and 1, 3, 5, 10, and 15 days postcardiotoxin-treated muscles. (B) Western blot analysis was performed to examine the steady-state protein levels of GEFT and the myogenic proteins, myosin heavy chain and myogenin, during cardiotoxin-induced regeneration. (C) Western blot analysis demonstrating sustained exogenous expression of GEFT in normal tibialis anterior muscles at 48 h and 15 days postinfection. (D) Immunofluorescence of GFP signal from infected and uninfected mouse tibialis anterior muscles taken 48 h and 15 days postinjection, further demonstrating the sustained expression of the AAV2/8-GEFT virus.
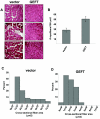
FIG. 2.
Sustained expression of GEFT in cardiotoxin-damaged tibialis anterior muscles induces muscle fiber regeneration. (A) H&E-stained sections of tibialis anterior muscle from control and GEFT AAV2/8-infected mice at 15 days after cardiotoxin-induced injury. (B) Number of myofibers per 100-μm2 area at 15 days postcardiotoxin injection. (C and D) The fiber cross-sectional area distribution of control and GEFT AAV2/8-infected tibialis anterior muscles measured at 15 days postcardiotoxin injection.
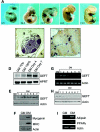
FIG. 3.
Expression of GEFT at mRNA and protein levels is highly regulated during myogenesis and adipogenesis. (A) Whole-mount in situ hybridizations of GEFT mRNA expression at mouse embryonic days 9.5 to 13.5. (B and C) Immunohistochemical detection of GEFT protein in the adult hind limb. Positive immunostaining appears brown (a, adipocytes; b, bone; s, skeletal muscle). Magnification for panel B, 40×; for panel C, 100×. (D) Northern blot analysis of GEFT steady-state mRNA expression in C2C12 cells subjected to proliferative growth medium (GM) at 70% and 100% confluence as well as at days 4 and 7 of differentiation conditions (DM). As a loading control, hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA expression was detected. (E) Western blot analysis of GEFT steady-state protein levels in C2C12 cells grown in growth medium (GM) or days 1 to 7 of differentiation (DM). (F) Analysis of myogenic markers by Western blot. To ensure that myogenic differentiation is properly occurring, Western blotting was performed in growth medium (GM) and day 5 differentiation (DM) for the myogenic markers myogenin and MHC. (G) RT-PCR detecting GEFT steady-state mRNA expression in 3T3L1 cells subjected to nondifferentiation (GM) and days 1 to 6 of differentiation (DM) conditions. (H) Western blot analysis of GEFT steady-state protein levels in 3T3L1 cells subjected to nondifferentiation (GM) and days 1 to 7 of differentiation (DM) conditions. (I) RT-PCR analysis of adipogenic differentiation in growth medium (GM) and day 4 differentiation (DM) for the adipogenic markers adipsin and PPARγ.
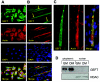
FIG. 4.
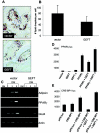
GEFT subcellular localization in C2C12 cells. Endogenous GEFT subcellular localization was detected by immunofluorescence with an anti-GEFT specific antibody in proliferative (A) and day 5 differentiation (B) conditions (400× magnification). (C) High-magnification image of GEFT subcellular localization in a multinucleated myotube. (D) Analysis of GEFT in cytoplasmic and nuclear fractions of C2C12 cells in growth medium (GM) and 4 days differentiation medium (DM). Western blot analysis was performed with an anti-GEFT specific antibody to confirm the subcellular localization of GEFT in cytoplasm. HDAC, histone deacetylase.
FIG. 5.
Regulation of RhoA, Rac1, Cdc42, and their downstream signaling pathways in C2C12 cells by exogenous expression of GEFT. (A) C2C12 cells were stably transfected with either a vector or GEFT plasmids. Cell lysates were collected and RhoA, Rac1, and Cdc42 were affinity purified by binding to GST-Rhotekin (for RhoA) or GST-Pak1 (for Rac1/Cdc42) beads. The protein bound to GST beads (active forms) as well as the total protein were analyzed by Western blotting with antibodies against RhoA, Rac1, or Cdc42. (B to F) Activation of downstream protein kinases by GEFT. Vector or GEFT stably transfected C2C12 cells were collected and the lysates were subjected to Western blot analysis with phosphospecific antibodies specific for the active, phosphorylated forms of (B) Rho kinase, (C) Pak1, (D) p38, (E) JNK, and (F) p42/44. As a loading control, Western blotting was performed with complementary antibodies (not phosphospecific) to determine the total levels of each protein. (G) Nuclear translocation of SRF promoted by GEFT. C2C12 cells were plated on glass coverslips and transiently cotransfected HA-tagged SRF with either an empty vector or FLAG-tagged GEFT. Immunofluorescent staining with anti-FLAG and anti-HA antibodies reveal the subcellular localization of SRF response to the presence or absence of exogenous GEFT.
FIG. 6.
GEFT strongly upregulates biomarkers of myogenic differentiation. (A and B) C2C12 cells, stably transfected with either vector (A) or GEFT (B) plasmids, were subjected to differentiation conditions. Photos were taken at day 3 of differentiation, showing a significant increase of myotubes in GEFT-expressing cells. (C) Histogram illustrating the mean number of myotubes per vision field in vector- and GEFT-transfected cells from growth media up to 5 days of differentiation. The data are the means ± standard errors of the means of at least three independent experiments. (D and E) Immunofluorescent staining with the myosin heavy chain-specific MF-20 antibody on vector (D) and GEFT (E) transfected C2C12 cells at day 2 of differentiation. Myosin heavy chain-positive cells appear green. (F) Graph illustrating the mean number of myosin heavy chain positive cells per vision field in vector- and GEFT-transfected cells from growth media up to 5 days differentiation. The data are means ± standard errors of the means of at least three independent experiments. (G) C2C12 cells stably transfected with either vector control or GEFT were differentiated, and cell lysates were collected at days 1, 2, and 3 of differentiation. Subsequent Western blot analysis was performed to detect the levels of different myogenic markers.
FIG. 7.
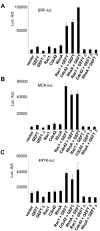
Regulation of myogenic transcription factors by the GEFT-mediated activation of the Rho proteins. C2C12 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the (A) muscle creatine kinase (MCK4800), (B) serum response factor (SRF), and (C) 4RTK promoter. Data are the means ± standard errors of the means of at least three independent experiments. Luc. Act., luciferase activity.
FIG. 8.
GEFT promotes the activation of MyoD and its heteromeric binding partners, E12 and E47. C2C12 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the muscle creatine kinase (MCK4800) promoter. Data are the means ± standard errors of the means of at least three independent experiments. Luc. Act., luciferase activity.
FIG. 9.
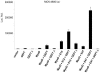
GEFT inhibits adipogenesis in 3T3L1 cells. (A) Oil Red O staining of vector- and GEFT-transfected 3T3L1 cells subjected to 5 days of differentiation. (B) Histogram illustrating the mean number of Oil Red O lipid droplets per cell in vector- and GEFT-transfected 3T3L1 cells at day 5 of differentiation. The data are means ± standard errors of the means of at least three independent experiments. P < 0.05 by Student’s t test. (C) 3T3L1 preadipocyte cells were stably transfected with vector control or GEFT. Cell lysates were collected in growth media (GM) and days 1, 3, and 5 of differentiation (DM). Subsequent RT-PCR analysis was performed for the adipogenic markers adipsin, PPARγ, and Glut4. (D and E) 3T3L1 cells were cotransfected with plasmids containing the luciferase reporter gene driven by the (D) PPARγ and (E) the CRE-BP1 promoter. Data are the means ± standard errors of the means of at least three independent experiments.
Similar articles
- A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration.
Guo X, Stafford LJ, Bryan B, Xia C, Ma W, Wu X, Liu D, Songyang Z, Liu M. Guo X, et al. J Biol Chem. 2003 Apr 11;278(15):13207-15. doi: 10.1074/jbc.M208896200. Epub 2003 Jan 23. J Biol Chem. 2003. PMID: 12547822 - GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation.
Bryan B, Kumar V, Stafford LJ, Cai Y, Wu G, Liu M. Bryan B, et al. J Biol Chem. 2004 Oct 29;279(44):45824-32. doi: 10.1074/jbc.M406216200. Epub 2004 Aug 17. J Biol Chem. 2004. PMID: 15322108 - p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene.
Lutz S, Freichel-Blomquist A, Rümenapp U, Schmidt M, Jakobs KH, Wieland T. Lutz S, et al. Naunyn Schmiedebergs Arch Pharmacol. 2004 May;369(5):540-6. doi: 10.1007/s00210-004-0926-5. Epub 2004 Apr 7. Naunyn Schmiedebergs Arch Pharmacol. 2004. PMID: 15069594 - The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.
Boissier P, Huynh-Do U. Boissier P, et al. Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review. - The Rho family of small GTPases: crucial regulators of skeletal myogenesis.
Bryan BA, Li D, Wu X, Liu M. Bryan BA, et al. Cell Mol Life Sci. 2005 Jul;62(14):1547-55. doi: 10.1007/s00018-005-5029-z. Cell Mol Life Sci. 2005. PMID: 15905962 Free PMC article. Review.
Cited by
- The Regulation of Cellular Responses to Mechanical Cues by Rho GTPases.
Hoon JL, Tan MH, Koh CG. Hoon JL, et al. Cells. 2016 Apr 6;5(2):17. doi: 10.3390/cells5020017. Cells. 2016. PMID: 27058559 Free PMC article. Review. - Genetic biomarkers in non-contact muscle injuries in elite soccer players.
Pruna R, Artells R, Lundblad M, Maffulli N. Pruna R, et al. Knee Surg Sports Traumatol Arthrosc. 2017 Oct;25(10):3311-3318. doi: 10.1007/s00167-016-4081-6. Epub 2016 Apr 16. Knee Surg Sports Traumatol Arthrosc. 2017. PMID: 27085366 - Rho GTPases in Skeletal Muscle Development and Homeostasis.
Rodríguez-Fdez S, Bustelo XR. Rodríguez-Fdez S, et al. Cells. 2021 Nov 2;10(11):2984. doi: 10.3390/cells10112984. Cells. 2021. PMID: 34831205 Free PMC article. Review. - Group I Paks support muscle regeneration and counteract cancer-associated muscle atrophy.
Cerquone Perpetuini A, Re Cecconi AD, Chiappa M, Martinelli GB, Fuoco C, Desiderio G, Castagnoli L, Gargioli C, Piccirillo R, Cesareni G. Cerquone Perpetuini A, et al. J Cachexia Sarcopenia Muscle. 2018 Aug;9(4):727-746. doi: 10.1002/jcsm.12303. Epub 2018 May 21. J Cachexia Sarcopenia Muscle. 2018. PMID: 29781585 Free PMC article. - The Popeye domain containing protein family--A novel class of cAMP effectors with important functions in multiple tissues.
Schindler RF, Brand T. Schindler RF, et al. Prog Biophys Mol Biol. 2016 Jan;120(1-3):28-36. doi: 10.1016/j.pbiomolbio.2016.01.001. Epub 2016 Jan 7. Prog Biophys Mol Biol. 2016. PMID: 26772438 Free PMC article. Review.
References
- Akimoto, T., T. Ushida, S. Miyaki, H. Akaogi, K. Tsuchiya, Z. Yan, R. S. Williams, and T. Tateishi. 2005. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem. Biophys. Res. Commun. 329:381-385. - PubMed
- Arnold, H. H., and T. Braun. 2000. Genetics of muscle determination and development. Curr. Top. Dev. Biol. 48:129-164. - PubMed
- Arnold, H. H., and B. Winter. 1998. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 8:539-544. - PubMed
- Arsic, N., S. Zacchigna, L. Zentilin, G. Ramirez-Correa, L. Pattarini, A. Salvi, G. Sinagra, and M. Giacca. 2004. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 10:844-854. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous