Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium - PubMed (original) (raw)
Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium
David H Broide et al. Proc Natl Acad Sci U S A. 2005.
Abstract
In response to inflammation or injury, airway epithelial cells express inducible genes that may contribute to allergen-induced airway remodeling. To determine the contribution of epithelial cell NF-kappaB activation to the remodeling response, we generated CC10-Cre(tg)/Ikkbeta(delta/delta) mice in which NF-kappaB signaling through IkappaB kinase beta (IKKbeta) is selectively ablated in the airway epithelium by conditional Cre-recombinase expression from the Clara cell (CC10) promoter. Repetitive ovalbumin challenge of mice deficient in airway epithelial IKKbeta prevented nuclear translocation of the RelA NF-kappaB subunit only in airway epithelial cells, resulting in significantly lower peribronchial fibrosis in CC10-Cre(tg)/Ikkbeta(delta/delta) mice compared with littermate controls as assessed by peribronchial trichrome staining and total lung collagen content. Levels of airway mucus, airway eosinophils, and peribronchial CD4+ cells in ovalbumin-challenged mice were also reduced significantly upon airway epithelial Ikkbeta ablation. The diminished inflammatory response was associated with reduced expression of NF-kappaB-regulated chemokines, including eotaxin-1 and thymus- and activation-regulated chemokine, which attract eosinophils and Th2 cells, respectively, into the airway. The number of peribronchial cells expressing TGF-beta1, as well as TGF-beta1 amounts in bronchoalveolar lavage, were also significantly reduced in mice deficient in airway epithelium IKKbeta. Overall, these studies show an important role for NF-kappaB regulated genes in airway epithelium in allergen-induced airway remodeling, including peribronchial fibrosis and mucus production.
Figures
Fig. 1.
Generation of mice lacking IKKβ in airway epithelium. Genotyping of CC10-Cretg/_Ikk_βΔ/Δ mice. CC10-Cretg mice (A) were crossed with Ikk_β_F/F mice (B) to generate, after doxycycline administration, CC10-Cretg/_Ikk_βΔ/Δ progeny in which the Ikk_β_F allele is selectively deleted in airway epithelium. (A1) In CC10-Cretg mice the CC10 promoter induces selective airway epithelial expression of rtTA. (A2) In the presence of doxycycline, rtTA binds to a _tetO_-containing promoter activating transcription of a transgene encoding Cre-recombinase in the airway epithelium. (B) Cre-recombinase cleaves at the loxP sites that flank exon 3 of the Ikk_β_F allele to produce the deleted _Ikk_βΔ allele, which is null for IKKβ expression. (C) RT-PCR analysis of CC10-Cretg/Ikk_β_F/F progeny (lane 3) indicates the presence of both transgenes contributed by the CC10-Cretg mice (lane 2), as well as the Ikk_β_F allele, contributed by the Ikk_β_F/F parents (lane 1). (D) TaqMan PCR studies showing that, in CC10-Cretg/_Ikk_βΔ/Δ mice, sequences specific to _Ikk_β exon 3 were absent in airway epithelial cells. (E) Activation of NF-κB after OVA challenge: Immunofluorescence microscopy of bronchiole sections stained with anti-RelA Abs and nuclear counterstaining with SYTOX green. RelA is cytoplasmic (green) in airway epithelial cells (arrow) as well as in peribronchial mononuclear cells of non-OVA-challenged WT and CC10-Cretg/_Ikk_βΔ/Δ mice. OVA-challenged WT mice exhibit nuclear RelA staining (yellow) in both airway epithelial cells (arrow) and peribronchial mononuclear cells. In OVA-challenged CC10-Cretg/_Ikk_βΔ/Δ mice RelA remained cytoplasmic in most airway epithelial cells (arrow) but was nuclear in peribronchial cells, which were reduced in number in CC10-Cretg/_Ikk_βΔ/Δcompared with WT mice.
Fig. 2.
Peribronchial fibrosis in CC10-Cretg/_Ikk_βΔ/Δ mice. WT and CC10-Cretg/_Ikk_βΔ/Δ mice were kept unchallenged or were subjected to repetitive OVA challenge. Lungs were collected, and the extent of fibrosis was evaluated by trichrome staining (blue).
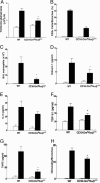
Fig. 3.
Quantitative analysis of airway fibrosis, TGF-β1, inflammation, mucus, and chemokines. WT and CC10-Cretg/_Ikk_βΔ/Δ mice were kept unchallenged or subjected to repetitive OVA challenge. (A) Lungs and BAL fluid were collected, and the extent of fibrosis was evaluated by lung trichrome staining and image analysis. (B) Lung mucus assessed by periodic acid/Schiff reagent staining. (C) BAL eosinophils revealed by Wright-Giemsa staining. (D) BAL eotaxin-1 measured by an ELISA. (E) BAL IL-5 measured by an ELISA. (F) Lung TGF-β1 measured by an ELISA. (G) BAL TARC measured by an ELISA. (H) Peribronchial CD4+ cells identified by immunostaining with an anti-CD4 Ab.
Similar articles
- Combination of corticosteroid therapy and allergen avoidance reverses allergen-induced airway remodeling in mice.
Cho JY, Miller M, McElwain K, McElwain S, Broide DH. Cho JY, et al. J Allergy Clin Immunol. 2005 Nov;116(5):1116-22. doi: 10.1016/j.jaci.2005.08.015. Epub 2005 Oct 10. J Allergy Clin Immunol. 2005. PMID: 16275385 - Immunostimulatory DNA reverses established allergen-induced airway remodeling.
Youn CJ, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Raz E, Broide DH. Youn CJ, et al. J Immunol. 2004 Dec 15;173(12):7556-64. doi: 10.4049/jimmunol.173.12.7556. J Immunol. 2004. PMID: 15585883 - Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13.
Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. Cho JY, et al. Respir Res. 2010 Nov 1;11(1):154. doi: 10.1186/1465-9921-11-154. Respir Res. 2010. PMID: 21040544 Free PMC article. - Cytokines and growth factors in airway remodeling in asthma.
Doherty T, Broide D. Doherty T, et al. Curr Opin Immunol. 2007 Dec;19(6):676-80. doi: 10.1016/j.coi.2007.07.017. Epub 2007 Aug 27. Curr Opin Immunol. 2007. PMID: 17720466 Review. - [Nuclear IκB-ζ controls inflammatory responses].
Maruyama T. Maruyama T. Seikagaku. 2015 Oct;87(5):601-4. Seikagaku. 2015. PMID: 26638629 Review. Japanese. No abstract available.
Cited by
- Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen.
Morgan CI, Ledford JR, Zhou P, Page K. Morgan CI, et al. J Inflamm (Lond). 2011 Dec 7;8:36. doi: 10.1186/1476-9255-8-36. J Inflamm (Lond). 2011. PMID: 22151973 Free PMC article. - Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation.
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Takahashi H, et al. Cancer Cell. 2010 Jan 19;17(1):89-97. doi: 10.1016/j.ccr.2009.12.008. Cancer Cell. 2010. PMID: 20129250 Free PMC article. - Transcriptional regulation of cytokine function in airway smooth muscle cells.
Clarke D, Damera G, Sukkar MB, Tliba O. Clarke D, et al. Pulm Pharmacol Ther. 2009 Oct;22(5):436-45. doi: 10.1016/j.pupt.2009.04.003. Epub 2009 Apr 22. Pulm Pharmacol Ther. 2009. PMID: 19393330 Free PMC article. Review. - Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm.
Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Fujisawa T, et al. J Immunol. 2009 Nov 15;183(10):6236-43. doi: 10.4049/jimmunol.0900614. Epub 2009 Oct 19. J Immunol. 2009. PMID: 19841186 Free PMC article. - Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease.
Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Edwards MR, et al. Pharmacol Ther. 2009 Jan;121(1):1-13. doi: 10.1016/j.pharmthera.2008.09.003. Epub 2008 Oct 6. Pharmacol Ther. 2009. PMID: 18950657 Free PMC article. Review.
References
- Cohn, L., Elias, J. A. & Chupp, G. L. (2004) Annu. Rev. Immunol. 22, 789-815. - PubMed
- Davies, D. E., Wicks, J., Powell, R. M., Puddicombe, S. M. & Holgate, S. T. (2003) J. Allergy Clin. Immunol. 111, 215-225. - PubMed
- Humbles, A. A., Lloyd, C. M., McMillan, S. J., Friend, D. S., Xanthou, G., McKenna, E. E., Ghiran, S., Gerard, N. P., Yu, C., Orkin, S. H. & Gerard, C. (2004) Science 305, 1776-1779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous