Morphodynamic profiling of protrusion phenotypes - PubMed (original) (raw)
Morphodynamic profiling of protrusion phenotypes
M Machacek et al. Biophys J. 2006.
Abstract
We propose a framework for tracking arbitrary complex cell boundary movements, relying on a unique definition of protrusion and retraction as the pathlength a virtual edge marker traverses when moving continuously perpendicular to the cell boundary. We introduce the level set method as a numerical scheme to reconstruct continuous boundary movement in time-lapse image sequences with finite time sampling. For moderately complex movements, we describe a numerically less expensive method that satisfactorily approximates the definition. Densely sampled protrusion and retraction rates were accumulated in space-time charts revealing distinct morphodynamic states. Applying this technique to the profiling of epithelial cell protrusion we identified three different states. In the I-state, long cell edge sectors are synchronized in cycles of protrusion and retraction. In the V-state random bursts of protrusion initiate protrusion waves propagating transversally in both directions. Cells switch between both states dependent on the Rac1 activation level. Furthermore, the persistence of transversal waves in the V-state depends on Arp2/3 concentration. Inhibition of PAK shifts cells into a lambda-state where continuous protrusion is occasionally interrupted by self-propagating ruffles. Our data support a model where activation of Rac1 mediates the propagation of protrusion waves, whose persistence depends on the relative abundance of activated Arp2/3 and polymerizable G-actin.
Figures
FIGURE 1
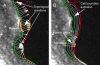
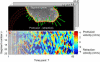
Problems of quantifying cell morphological changes (A_–_C). A frequently used measure of morphological changes is the cell area difference A between two time points. This parameter cannot capture local variations in the cell shape and thus is incapable of distinguishing many obvious morphodynamic phenotypes. (D) Morphological changes quantified by pointwise measurement of boundary displacements. The main difficulty of this method is defining the direction of displacement. In areas of strong boundary deformation, the normal direction is not meaningful (arrowhead 1) and any other choice is arbitrary (arrowhead 2). (E) Morphological changes in polar coordinates are measured by length variations of the radial coordinate r. For noncircular cell outlines this measurement is not unique (arrowhead 1) and the spatial resolution of the measurement varies substantially (arrowhead 2). (F) We propose a coordinate system based on the run length s of the boundary to avoid the polar coordinate-associated problems. Image courtesy of P. Nalbant.
FIGURE 2
Definition of terms used for the morphodynamic measurements proposed in this article. (Markers) Virtual particles placed on the boundary at time point T. (Marker path) Trajectory of a marker between T and T + 1. Multiplying marker pathlength (displacement) with the frame rate yields the protrusion and retraction velocities. The difficulty of morphodynamic measurements is finding a rule to obtain consistent marker paths for all boundary shapes. (A) Following the normal direction, marker paths can cross (topological violation). (B) Topological violation is avoided by using continuous normal propagation of the cell boundary to find consistent marker paths between T and T + 1.
FIGURE 3
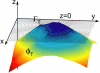
The principal idea of the level set method. The cell boundary is embedded in the level set function
defined as the distance function of the boundary. To visualize the level set function
the distance from the cell outline is plotted on the z axis. The entire level set is propagated along its gradient
according to Eq. 1 and evolved into the level set
embedding the cell boundary
See Movie 1 in the Supplementary Material for an animated evolution sequence of the level set function
and its corresponding zero level curve
FIGURE 4
Level set algorithm.
FIGURE 5
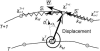
Measurement of boundary displacement vector () by the mechanical method. The position of the marker
is determined by the equilibrium of linear spring forces
and a torsion spring force
. The torsion spring enforces normal displacement in the direction
. The linear springs enforce regular spacing while maintaining the sequence order (1,..,i_−1,i,i+1,…_N) of markers (topological consistency).
FIGURE 6
Evaluation of the LSM on two test cases using three different speed functions. The computational grid size was (3,3) pixels and the adaptable time step of the integrator was on average 0.1. See text for a discussion of the difference in speed functions.
FIGURE 7
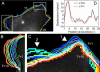
Evaluation of the LSM and the mechanical method on migrating cells. (A_–_F) Analysis of whole-cell movement of a MEF. Spatial resolution, 314 nm/pixel. Frame interval, 1 min. Image courtesy of O. Pertz. (A) Marker paths obtained from the LSM. (B) Displacement vectors obtained by the mechanical model. In the area of high boundary deformation (arrowhead 1), the method fails to deliver a topologically consistent solution. (C) Comparison of the two methods shows only small differences in the protrusion and retraction rates, despite the topological violation by the mechanical model. (D) Boundary evolution obtained by the LSM for the same cell but with a frame interval of 10 min. (E) Marker paths. (F) Detail showing the marker paths for a region of high boundary deformation. To accommodate the strong contraction of the cell membrane during retraction the marker paths became strongly curved and converged. (G,H) High-resolution analysis of the protruding boundary of a PtK1 cell. Spatial resolution, 67 nm/pixel. Frame interval, 10 s. (G) Displacement vectors obtained by the mechanical method. (Inset) Displacements of <1 pixel were resolved. (H) Comparison of protrusion and retraction rates obtained by LSM and mechanical method.
FIGURE 8
Evaluation of the LSM edge interpolation. (A) Segmented boundaries from 10 time points of a migrating MEF. Cell outlines are time color coded. (blue) Early time points; (red) late time points. Frame interval, 20 s. (B) Detail of a boundary section with unidirectional motion. The boundary evolution from T = 1 to T = 10 obtained from the LSM (white lines) overlaid on the segmented boundaries at T_=1,2…9,10. (C) Detail of a boundary section with switching between protrusions and retractions. (D) Displacements of the cell boundary from T = 1 until T = 10, measured once using a frame interval of Δ_T = 1 (black curve) and once using a frame interval of Δ_T_ = 9 (red curve). Image courtesy of O. Pertz.
FIGURE 9
Construction of morphodynamic activity maps. Boundary displacements between frames 1 and 2 were filled into the first column of the activity map. The same procedure was repeated for the following time steps. The color at (s,T) encodes the displacement velocity at time point T and boundary segment s. To reduce the noise, activity maps were filtered in the time direction using smoothing splines with a smoothing factor corresponding to a Gaussian filter width of ∼2 time steps.
FIGURE 10
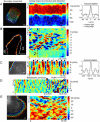
Comparison of morphodynamic patterns in different cell types. (A) Activity map of a migrating keratocyte indicating a persistent polarity of the leading and trailing edge. Cell protrusion at the leading is uniform, whereas retraction at the trailing edge occurs with a left-right periodicity (circles). Scale bar = 10 _μ_m. Autocorrelation of the morphodynamics of leading edge and trailing edge. Image courtesy of P. Yam, C. Wilson, and J. Theriot. (B) MEFs exhibited a distinct and persistent separation of active (bracket 1) and quiescent (bracket 2) boundary regions. Activity maps revealed transversal protrusion waves traveling with constant speed along the cell boundary (dashed lines). Scale bar = 20 _μ_m. Image courtesy of O. Pertz. (C) Leading edge protrusion of a NLE cell. The boundary was oscillating in a coordinated fashion (I-state) with a remarkably constant frequency of 100 s (cf. autocorrelation). Scale bar = 3 _μ_m. (D) Leading edge protrusion of a NLE cell before and after perfusion with blebbistatin. Waiting time after perfusion before morphological profiling was 20 min. The drug treatment had no effect on the morphodynamic state of the NLE cell and did not markedly change the periodicity of the I-state. Image courtesy of C. Waterman-Storer. (E) Leading edge protrusion of a PtK1 epithelial cell. Morphodynamic patterns resulted from a superposition of transversal protrusion waves traveling with constant speed along the boundary (V-state). Scale bar = 3 _μ_m. Image courtesy of J. Lim.
FIGURE 11
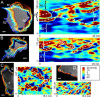
Comparison of protrusion and retraction activity maps from MEFs to demonstrate low variability in morphodynamic patterns despite significantly different cell outline shapes. (A,B) Activity maps of entire cells. Leading edge of the cell is indicated with P and trailing edge with R. Transversal wave propagation speeds are 4.2 and 6.3 _μ_m/min (movies 5 and 6). (C,D) Activity maps of the leading edge of polarized, but slowly migrating MEFs. Note that the leading edge activity is the same as for the migrating cells. Transversal wave propagation speeds 12 and 8.2 _μ_m/min (movies 7 and 8). Quiescent region is indicated with Q. Scale bars = 10 _μ_m.
FIGURE 12
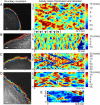
Perturbation analysis of morphodynamic patterns in PtK1 epithelial cells. (A) Control cell characterized by V-state. Image courtesy of J. Lim. (B) PtK1 cell expressing constitutively active Rac1(Q61L). It phenocopies the I-state of NLE cells (cf. Fig. 10 C). Image courtesy of T. Wittmann. (C) PtK1 cell expressing Rac1(Q61L) and with PAK inhibition. The cell turns into a hyperactivated protrusion state mediated by permanent actin polymerization. This protrusion state is only interrupted by transversally propagating ruffles leading to short-term retraction of the edge. Image courtesy of V. Delorme. (D,E) PtK1 cell with inhibited Arp2/3. This intervention maintains the V-state but fewer and less persistent waves are initialized. Bracket 1 indicated typical wave persistence duration of 2 min. The propagation speed of these waves was not affected by Arp2/3 inhibition. Image courtesy of S. Gupton. Scale bar = 3 _μ_m.
FIGURE 13
Mechanism of Rac1 dependent propagation of protrusion waves. A random burst of polymerization initiates protrusion waves propagating transversally in both directions. The waves are self-sustained by a feedback mechanism where polymerization pressure activates Rac1 at adhesion sites, which triggers Arp2/3 mediated polymerization. Polymerization pressure exerts a force onto the actin network. Due to network viscoelastic properties, the load is distributed onto adhesion complexes not only right behind the protrusion region but also in a lateral vicinity, leading to lateral propagation of the feedback mechanism. Locally polymerization lasts until resources are depleted. After critical resources for polymerization are renewed in the depleted region a new wave can be initiated. This protrusion propagation mode leads to the V-state as observed in the experiments (cf. Fig. 10 D).
Similar articles
- ERK reinforces actin polymerization to power persistent edge protrusion during motility.
Mendoza MC, Vilela M, Juarez JE, Blenis J, Danuser G. Mendoza MC, et al. Sci Signal. 2015 May 19;8(377):ra47. doi: 10.1126/scisignal.aaa8859. Sci Signal. 2015. PMID: 25990957 Free PMC article. - Cdc42 is required for EGF-stimulated protrusion and motility in MTLn3 carcinoma cells.
El-Sibai M, Nalbant P, Pang H, Flinn RJ, Sarmiento C, Macaluso F, Cammer M, Condeelis JS, Hahn KM, Backer JM. El-Sibai M, et al. J Cell Sci. 2007 Oct 1;120(Pt 19):3465-74. doi: 10.1242/jcs.005942. Epub 2007 Sep 12. J Cell Sci. 2007. PMID: 17855387 Free PMC article. - Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion.
DeMali KA, Barlow CA, Burridge K. DeMali KA, et al. J Cell Biol. 2002 Dec 9;159(5):881-91. doi: 10.1083/jcb.200206043. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473693 Free PMC article. - Requirements for and consequences of Rac-dependent protrusion.
Steffen A, Koestler SA, Rottner K. Steffen A, et al. Eur J Cell Biol. 2014 May-Jun;93(5-6):184-93. doi: 10.1016/j.ejcb.2014.01.008. Epub 2014 Feb 11. Eur J Cell Biol. 2014. PMID: 24629839 Review. - A role for the small GTPase Rac1 in vaccinia actin-based motility.
Alvarez DE, Agaisse H. Alvarez DE, et al. Small GTPases. 2015;6(2):119-22. doi: 10.1080/21541248.2015.1055182. Small GTPases. 2015. PMID: 26147090 Free PMC article. Review.
Cited by
- Short-term molecular polarization of cells on symmetric and asymmetric micropatterns.
Kandere-Grzybowska K, Soh S, Mahmud G, Komarova Y, Pilans D, Grzybowski BA. Kandere-Grzybowska K, et al. Soft Matter. 2010 Jul 21;6(14):3257-3268. doi: 10.1039/B922647H. Soft Matter. 2010. PMID: 23826026 Free PMC article. - Cell motility: The necessity of Rac1 GDP/GTP flux.
Parrini MC, Camonis J. Parrini MC, et al. Commun Integr Biol. 2011 Nov 1;4(6):772-4. doi: 10.4161/cib.17772. Commun Integr Biol. 2011. PMID: 22446552 Free PMC article. - Directional persistence of cell migration coincides with stability of asymmetric intracellular signaling.
Weiger MC, Ahmed S, Welf ES, Haugh JM. Weiger MC, et al. Biophys J. 2010 Jan 6;98(1):67-75. doi: 10.1016/j.bpj.2009.09.051. Biophys J. 2010. PMID: 20085720 Free PMC article. - Protocol for live cell image segmentation to profile cellular morphodynamics using MARS-Net.
Jang J, Hallinan C, Lee K. Jang J, et al. STAR Protoc. 2022 Jun 14;3(3):101469. doi: 10.1016/j.xpro.2022.101469. eCollection 2022 Sep 16. STAR Protoc. 2022. PMID: 35733606 Free PMC article. - Propagating cell-membrane waves driven by curved activators of actin polymerization.
Peleg B, Disanza A, Scita G, Gov N. Peleg B, et al. PLoS One. 2011 Apr 21;6(4):e18635. doi: 10.1371/journal.pone.0018635. PLoS One. 2011. PMID: 21533032 Free PMC article.
References
- Wessels, D., and D. R. Soll. 1998. Computer-assisted characterization of the behavioral defects of cytoskeletal mutants of Dictyostelium discoideum. In Motion Analysis of Living Cells. Wiley-Liss, Hoboken, NJ. 101–140.
- Heid, P. J., J. Geiger, D. Wessels, E. Voss, and D. R. Soll. 2005. Computer-assisted analysis of filopod formation and the role of myosin II heavy chain phosphorylation in Dictyostelium. J. Cell Sci. 118:2225–2237. - PubMed
- Waterman-Storer, C. M., R. A. Worthylake, B. P. Liu, K. Burridge, and E. D. Salmon. 1999. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1:45–50. - PubMed
- Dunn, G. A., and D. Zicha. 1995. Dynamics of fibroblast spreading. J. Cell Sci. 108:1239–1249. - PubMed
- Zicha, D., I. M. Dobbie, M. R. Holt, J. Monypenny, D. Y. H. Soong, C. Gray, and G. A. Dunn. 2003. Rapid actin transport during cell protrusion. Science. 300:142–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials