Off-line learning of motor skill memory: a double dissociation of goal and movement - PubMed (original) (raw)
Comparative Study
. 2005 Dec 13;102(50):18237-41.
doi: 10.1073/pnas.0506072102. Epub 2005 Dec 5.
Affiliations
- PMID: 16330773
- PMCID: PMC1312380
- DOI: 10.1073/pnas.0506072102
Comparative Study
Off-line learning of motor skill memory: a double dissociation of goal and movement
Daniel A Cohen et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Acquiring a new skill requires learning multiple aspects of a task simultaneously. For example, learning a piano sonata requires learning the musical notes and being able to implement this goal by learning the appropriate sequence of finger movements. After practice, skill continues to develop off-line during a period of consolidation. Here we show that different aspects of a procedural memory are processed separately during consolidation: Only the movement sequence is enhanced over the day; whereas only the goal is enhanced over a night of sleep. This double dissociation suggests that distinct systems, enhancing different aspects of a procedural memory, support improvements during consolidation. Consolidation is not a single process; instead, there are multiple routes to off-line learning, and the engagement of these distinct mechanisms is determined by when consolidation takes place.
Figures
Fig. 1.
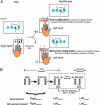
Experimental design. (A) Design used to dissociate goal- and movement-based skill improvements. Visual cues presented on a screen guide the acquisition of skill during practice. Skill in this task is due to learning a series of finger movements (e.g., -middle-little-ring) combined with learning a sequence of response buttons (e.g., -2-4-3), or goals (2). Switching hands makes it possible to distinguish between these skill components: (i) maintaining the goal (e.g., -2-4-3) but altering the order of finger movements (goal configuration) measures the skill derived from knowledge of the goal (i.e., knowledge of the sequence, independent of the fingers used), whereas (ii) maintaining the order of finger movements (e.g., -middle-little-ring) but altering the goal (movement configuration) measures the skill derived from the finger movements (i.e., knowledge of the specific finger movements, independent of the sequence of response buttons). This later type of manipulation produces a mirror sequence [e.g., from -2-4-3 to -3-1-2, (7, 27)]. (B) The first session consisted of a single training block sandwiched between two test blocks. A participant's skill (skill1) was “probed” by using either the goal or movement configuration of the task. During the second session, 12 or 24 h later, the same skill component was probed by using a single test block. Within each block, sequential trials (white blocks) were sandwiched between random trials (gray blocks). A standard measure of skill in this task is to calculate the difference between the response times of the sequential and the following random trials (10, 12, 15). During the first session, participants initially performed the task with their right hand (RightStandard), before switching to their left hand for the test block. This manipulation allowed the skill acquired with the right hand to be decomposed into goal- and movement-based components. Switching hands can alter the sequence of finger movements used to achieve the same sequence of spatial goals (LeftStandard), providing a measure of goal-based skill. Alternatively, transforming a standard sequence into a mirror sequence (e.g., from -2-4-3 to -3-1-2) alters the sequence of spatial goals but preserves the finger movement sequence (LeftMirror), providing a measure of movement-based skill.
Fig. 2.
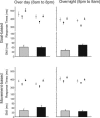
Goal- or movement-based skill was tested (skill1, gray boxes) and retested (skill2, black boxes) after a 12-h interval with (8 p.m. to 8 a.m.) or without (8 a.m. to 8 p.m.) sleep. At initial testing, there was no significant difference in skill across the four groups [F(3, 36) = 1.142, P = 0.345]. However, there was a differential off-line improvement in the skill components across the interval: Goal-based skill only improved over a night of sleep, whereas movement-based skill improved only over the day. Across the interval, there was also a general improvement in performance, shown by the fall in response time to the random trials (gray triangle ± SE), which preceded the sequential trials [paired t test, t(39) = 4.9, P < 0.001]. This fall in response time did not differ significantly across the four groups [F(3, 36) = 0.758, P = 0.525]. These general performance improvements are factored out when using the standard measure of skill for this task, the difference between response times to sequential trials (black square ± SE), and the following random (black circle ± SE) trials (10, 12, 15). When random trials are introduced after sequential trials (postrandom trials), participants continue to play out the sequence. This produces proactive interference from the sequential to the random trials. The amount of this interference remains unchanged at testing and retesting when there is no off-line learning (over day/goal and overnight/movement). In these groups, there is a significant decrease in the response time to the postrandom trials between testing and retesting [paired t test, t(19) = 3.5, P = 0.002]. This reflects the general improvement in task performance. In contrast, the effects of increased proactive interference, which result from an enhancement of sequence specific skill off-line (over day/movement and overnight/goal) counteract this general performance improvement. Consequently, there is no significant change in the response time to the postrandom trials between testing and retesting in those groups that show off-line improvements [paired t test, t(19) = 0.426, P = 0.675].
Fig. 3.
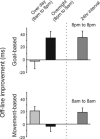
Goal-based improvements develop only overnight, whereas movement-based improvements develop only over day. Off-line skill improvements (with SEs) are shown. Goal- or movement-based skill was tested before (skill1) and retested after (skill2) an interval of 12 h (main experimental groups to the left of the dotted line) or 24 h (diurnal control groups to the right of the dotted line). The skill at testing and at retesting in the main experimental groups is shown in Fig. 2. For all groups, the difference between these measures (skill2 - skill1) is shown above. Where the difference is significantly greater than zero, off-line learning had occurred. Goal-based improvements were observed only when the interval included a period of sleep (8 p.m. to 8 a.m.). These improvements are not coupled to a particular time of day, because they can still be observed in the evening (8 p.m. to 8 p.m.). Movement-based improvements were observed over day (8 a.m. to 8 p.m.) but not overnight (8 p.m. to 8 a.m.). These improvements were also not coupled to a particular time of day, because they could still be observed in the morning (8 a.m. to 8 a.m.). This double dissociation suggests that off-line learning can be supported by distinct mechanisms enhancing different aspects of a procedural memory.
Similar articles
- Motor skill learning and offline-changes in TGA patients with acute hippocampal CA1 lesions.
Döhring J, Stoldt A, Witt K, Schönfeld R, Deuschl G, Born J, Bartsch T. Döhring J, et al. Cortex. 2017 Apr;89:156-168. doi: 10.1016/j.cortex.2016.10.009. Epub 2016 Oct 24. Cortex. 2017. PMID: 27890324 - Off-line processing: reciprocal interactions between declarative and procedural memories.
Brown RM, Robertson EM. Brown RM, et al. J Neurosci. 2007 Sep 26;27(39):10468-75. doi: 10.1523/JNEUROSCI.2799-07.2007. J Neurosci. 2007. PMID: 17898218 Free PMC article. - Off-line learning and the primary motor cortex.
Robertson EM, Press DZ, Pascual-Leone A. Robertson EM, et al. J Neurosci. 2005 Jul 6;25(27):6372-8. doi: 10.1523/JNEUROSCI.1851-05.2005. J Neurosci. 2005. PMID: 16000627 Free PMC article. - Does sleep promote motor learning? Implications for physical rehabilitation.
Siengsukon CF, Boyd LA. Siengsukon CF, et al. Phys Ther. 2009 Apr;89(4):370-83. doi: 10.2522/ptj.20080310. Epub 2009 Feb 6. Phys Ther. 2009. PMID: 19201986 Review. - Understanding consolidation through the architecture of memories.
Robertson EM, Cohen DA. Robertson EM, et al. Neuroscientist. 2006 Jun;12(3):261-71. doi: 10.1177/1073858406287935. Neuroscientist. 2006. PMID: 16684970 Review.
Cited by
- Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory.
Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J. Albouy G, et al. PLoS One. 2013;8(1):e52805. doi: 10.1371/journal.pone.0052805. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23300993 Free PMC article. - Neural plasticity and its contribution to functional recovery.
Sharma N, Classen J, Cohen LG. Sharma N, et al. Handb Clin Neurol. 2013;110:3-12. doi: 10.1016/B978-0-444-52901-5.00001-0. Handb Clin Neurol. 2013. PMID: 23312626 Free PMC article. Review. - Cued Reactivation of Motor Learning during Sleep Leads to Overnight Changes in Functional Brain Activity and Connectivity.
Cousins JN, El-Deredy W, Parkes LM, Hennies N, Lewis PA. Cousins JN, et al. PLoS Biol. 2016 May 3;14(5):e1002451. doi: 10.1371/journal.pbio.1002451. eCollection 2016 May. PLoS Biol. 2016. PMID: 27137944 Free PMC article. - Human relational memory requires time and sleep.
Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Ellenbogen JM, et al. Proc Natl Acad Sci U S A. 2007 May 1;104(18):7723-8. doi: 10.1073/pnas.0700094104. Epub 2007 Apr 20. Proc Natl Acad Sci U S A. 2007. PMID: 17449637 Free PMC article. - Time of day and sleep effects on motor acquisition and consolidation.
Truong C, Ruffino C, Gaveau J, White O, Hilt PM, Papaxanthis C. Truong C, et al. NPJ Sci Learn. 2023 Sep 1;8(1):30. doi: 10.1038/s41539-023-00176-9. NPJ Sci Learn. 2023. PMID: 37658041 Free PMC article.
References
- Brooks, V. B. (1986) The Neural Basis of Motor Control (Oxford Univ. Press, New York).
- Willingham, D. (1999) Mem. Cognit. 27, 561-572. - PubMed
- Willingham, D., Wells, L., Farrell, J. & Stemwedel, M. (2000) Mem. Cognit. 28, 366-375. - PubMed
- Japikse, K., Negash, S., Howard, J. & Howard, D. (2003) Exp. Brain Res. 148, 38-49. - PubMed
- Hikosaka, O., Nakamura, K., Sakai, K. & Nakahara, H. (2002) Curr. Opin. Neurobiol. 12, 217-222. - PubMed
Publication types
MeSH terms
Grants and funding
- MH-65434/MH/NIMH NIH HHS/United States
- K30 HL04095/HL/NHLBI NIH HHS/United States
- RR018875/RR/NCRR NIH HHS/United States
- K23 MH065434/MH/NIMH NIH HHS/United States
- K30 HL004095/HL/NHLBI NIH HHS/United States
- K24 RR018875/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical