Autoantibodies make a U-turn: the toll hypothesis for autoantibody specificity - PubMed (original) (raw)
Review
Autoantibodies make a U-turn: the toll hypothesis for autoantibody specificity
David A Martin et al. J Exp Med. 2005.
Abstract
Like the immune response itself, our efforts to understand the "rules" for self-nonself discrimination are constantly evolving. The discovery of pattern recognition receptors-the Toll-like receptor (TLR) family in particular-shifted the emphasis of self-nonself recognition from lymphocytes functioning in the adaptive immune system to antigen-presenting cells (APCs) functioning in the innate immune system. Two new articles, one in a recent issue (1) and one in this issue (see Vollmer et al. [2] on p. 1575), demonstrate that antigen-antibody complexes containing RNAs activate B lymphocytes and dendritic cells (DCs) through interaction with TLR7 and/or TLR8. From these and other papers, one begins to see how specific types of autoantigens-by virtue of their capacity to act as TLR ligands-favor autoantibody production. This is known as the Toll hypothesis.
Figures
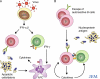
Figure 1.
Possible mechanisms by which nucleoprotein antigens initiate autoantibody production. (A) A virus infection is sensed by TLRs in plasmacytoid dendritic cells (pDCs) resulting in the production of large concentrations of type I interferon (IFN-α/β). IFNs prime the adaptive immune system to respond to other signals that may include nucleoprotein antigens released from dead and dying cells. Virus persistence and/or defective clearance of apoptotic cells might drive chronic, self-perpetuating autoimmunity through uptake of nucleoprotein antigen–antibody complexes (Fig. 2). (B) Defective B cell tolerance leads to capture of nucleoprotein antigens by autoreactive B cells, thereby triggering B cell activation, TLR stimulation, and antigen presentation to T cells. Apoptotic cells are abundant in germinal centers, and nucleoprotein antigens may be released at other sites due to abnormal cell death or defective cell clearance.
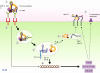
Figure 2.
Possible mechanisms by which engagement of the U1 snRNP antigen activates APCs. (1) The U1 snRNP binds to the BCR on a B cell or FcγR on a DC. (2) Cross-linking of the receptor results in an activation signal. (3) The receptor is endocytosed, and the endosome matures into a late endosome/lysosome containing TLR7 and 8. (4) The U-rich RNA, initially protected by the SmRNP proteins and/or the antibody, now engages the TLR triggering activation of IRF and NF-κB signal transduction pathways. (5) The protein component dissociates and is degraded by lysosomal enzymes with membrane fusion to vesicles containing MHC class II. (6) Activated transcription factors induce expression of proinflammatory cytokines such as type I IFNs, IL-12, IL-6, and TNF which (7) up-regulate MHC class II and costimulatory molecule expression. Cell activation may also lead to cell proliferation.
References
- Lau, C.M., C. Broughton, A.S. Tabor, S. Akira, R.A. Flavell, M.J. Mamula, S.R. Christensen, M.J. Shlomchik, G.A. Viglianti, I.R. Rifkin, and A. Marshak-Rothstein. 2005. RNA-associated autoantigens activate B cells by combined B cell receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171–1177. - PMC - PubMed
- Leadbetter, E.A., I.R. Rifkin, A.M. Hohlbaum, B.C. Beaudette, M.J. Shlomchik, and A. Marshak-Rothstein. 2002. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–607. - PubMed
- Diebold, S.S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303:1529–1531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous