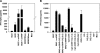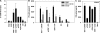Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8 - PubMed (original) (raw)
Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8
Jörg Vollmer et al. J Exp Med. 2005.
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies to certain cellular macromolecules, such as the small nuclear ribonucleoprotein particles (snRNPs), which had been considered to be passive targets of the autoimmune response. SLE is also characterized by the increased expression of type I interferon (IFN), which appears to be associated with the development and severity of disease. Here, we show that specific, highly conserved RNA sequences within snRNPs can stimulate Toll-like receptors (TLRs) 7 and 8 as well as activate innate immune cells, such as plasmacytoid dendritic cells (pDCs), which respond by secreting high levels of type I IFN. SLE patient sera containing autoantibodies to snRNPs form immune complexes that are taken up through the Fc receptor gammaRII and efficiently stimulate pDCs to secrete type I IFNs. These results demonstrate that a prototype autoantigen, the snRNP, can directly stimulate innate immunity and suggest that autoantibodies against snRNP may initiate SLE by stimulating TLR7/8.
Figures
Figure 1.
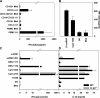
U1 snRNP induces type I IFN production. (A) Human PBMCs were stimulated with U1 snRNP (concentration given for total RNA plus protein) complexed to DOTAP (DO), 100 ng/ml LPS, or DOTAP alone, and cytokines were measured. Med, medium control. (B) SDS page of U1 snRNP, 4% stacking, 13.5% separation gel under reducing conditions, and Coomassie blue staining. Lane UF, RNP/Sm antigen, 8.8 μg protein; lane M, SigmaMarker, wide molecular weight range. (C) PBMCs were cultured with 20 μg/ml snRNP, 10 μg/ml poly rI:rC (pIC), snRNP, or poly rI:rC pretreated with RNase A or snRNP pretreated with proteinase K complexed to DOTAP. (D) PBMCs were stimulated with U1 snRNP or U1 snRNA complexed to DOTAP or DOTAP alone. The stimulatory properties of U1 snRNP or snRNA required the presence of an uptake enhancer (not depicted). All experiments show mean ± SEM of one representative out of two or more experiments, each with at least three to six donors.
Figure 2.
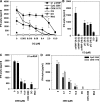
TLR7 is required for the immune response to U1 snRNP. (A) Human PBMCs, CD14-enriched PBMCs (CD14+), CD14- and CD123-depleted PBMCs (CD14− CD123−), or CD123-enriched PBMCs (CD123+) were stimulated with 20 μg/ml U1 snRNP complexed to DOTAP (DO), and cytokines were measured. Med, medium control. Mean ± SEM of three donors. (B) Murine RAW246 macrophages were stimulated for 16 h with 20 μg/ml U1 snRNP complexed to DOTAP, 1.0 μM CpG ODN 1826, or DOTAP alone. (C–D) Murine DCs derived from Flt3L-induced bone marrow cultures from C57/B6 wild-type or TLR7−/− mice were cultured with 20 μg/ml U1 snRNP or ORN 1170 or ORN 1177 alone or complexed to DOTAP, 2 μg/ml R-848, or 1 μM CpG ODN 1668 or ODN 2216. Percentage of pDCs was 44% for wild type and 45% for TLR7−/− bone marrow (measured as CD45RA and CD11chigh cells).
Figure 3.
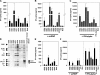
U1 snRNA ORNs stimulate cytokine production and TLR8 signaling. PBMCs were stimulated with DOTAP alone (DO) or complexed to ORN at the indicated concentrations (A, E, and H) at 0.25 μM (C, D and F) or 0.5 μM (B and D), and cytokines were measured. Med, medium control. Panels show one representative of up to three independent experiments (each with three donors; mean ± SEM) or one representative of three donors (E and H; mean ± SD). (I) hTLR8-HEK293 cells were incubated with 3 μM ORN complexed to DOTAP, 10 μM R-848, or 10 μM CpG ODN for 16 h. Stimulation indices represent -fold NF-κB activation compared with transfected, nonstimulated cells. (J) hTLR8-HEK293 cells were incubated with 10 μM ORN complexed to DOTAP for 16 h.
Figure 4.
U1 snRNA ORNs induce cytokine secretion from purified human monocytes, pDCs, or mDCs. Human PBMCs, CD14-enriched PBMCs (CD14+), CD14- and CD123-depleted PBMCs (CD14− CD123−), CD123- enriched PBMCs (CD123+), CD19- and CD1c-depleted PBMCs (CD14− BDCA-1−), or CD1c-enriched PBMCs (BDCA-1+) were stimulated with 0.5 μM ORN 1170 and/or 1271 complexed to DOTAP (DO), DO alone, or 0.5 μM CpG ODN and tested for (A) IFN-α, (B and D) TNF-α, and (C) IL-12p40 secretion. Mean ± SEM of two (A) or three (B–D) donors. Med, medium control.
Figure 5.
Chloroquine and S-Class ODN 2088 block the immune stimulatory effects of U1 snRNP and U1-derived ORNs. (A and B) Human PBMCs were stimulated with medium (Med), 10 μg/ml U1 snRNP complexed to DOTAP (DO), 0.5 μM CpG ODN 2395, 0.125 μM ORN 1170 complexed to DOTAP, or 30 μg/ml poly rI:rC (pIC) alone or in the presence of the indicated concentrations of chloroquine (CQ) or (C and D) S-Class ODN 2088, or with 10 μg/ml of ODN 2088 alone. ODN 2088 also inhibited ORN-induced TNF-α secretion (not depicted). Mean ± SEM of one representative out of two to three independent experiments (n = 3 donors).
Figure 6.
SLE patient sera containing anti-RNP antibodies combine with purified U1 snRNP to induce IFN-α production. (A and B) Normal human PBMCs were stimulated in 87.5% culture medium with 12.5% of eight (A–C) or six (E and F) SLE patient sera without (A and E) or with (B and F) 10 μg/ml U1 snRNP, or were pretreated with 500 IU/ml IFN-α2b (C and F) and assayed for IFN-α. Controls included medium (Med) and DOTAP (DO) alone or with 10 μg/ml U1 snRNP. Mean ± SEM for three donors. (D) Eight coded SLE patient sera (for all sera see Table II) were tested for the presence and level of various autoantibodies, including RNP components by radioimmunoprecipitation. Assessment of anti-RNP levels within these eight sera revealed two strongly positive sera (6571 and 6574), two moderate positive (6564 and 6579), and one very weak positive (6576), with the others negative.
Figure 7.
U1 snRNP and anti-RNP**+** SLE patient sera stimulate RNA-dependent IFN-α production inhibited by chloroquine and bafilomycin. (A) SLE patient sera 6622 and 6628 with 10 μg/ml U1 snRNP pretreated with RNase A were used to stimulate human PBMCs as described in Fig. 6. Mean ± SEM for three donors. (B) Normal human PBMCs were stimulated with SLE serum 6642 alone or with 10 μg/ml U1 snRNP or 0.5 μM CpG ODN 2395 in the presence or absence of 1.0 or 5.0 μM chloroquine (CQ) or 500 nM bafilomycin (Baf). Controls included chloroquine or bafilomycin alone or 0.5 μM ORN 1170. Mean ± SEM for two donors.
Figure 8.
Anti-RNP**+** SLE patient sera and U1 snRNP stimulate monocyte maturation and CD32-dependent IFN-α production from pDCs. (A) PBMCs were stimulated with SLE patient sera 6622 and 6628 with 10 μg/ml U1 snRNP, snRNP complexed to DOTAP (DO), or with 0.5 μM CpG ODN 2395 as described in Fig. 6. CD80 expression on CD14+ cells was measured by flow cytometry. Mean ± SEM for two donors. (B) Whole PBMCs, CD14- and CD123-depleted PBMCs (CD14− CD123−), or purified CD123+ pDCs were cultured as shown. Mean ± SEM for three donors. (C) Purified CD123+ pDCs were incubated with SLE patient sera 6571 (or 6574; not depicted) and 10 μg/ml U1 snRNP, or 0.5 μM of positive control CpG ODN 2395 ± 5 μg/ml antibody to CD16 or CD32. Mean ± SD for one out of two experiments.
Similar articles
- Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjögren's syndrome autoantigen-associated RNA.
Lövgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Rönnblom L. Lövgren T, et al. Arthritis Rheum. 2006 Jun;54(6):1917-27. doi: 10.1002/art.21893. Arthritis Rheum. 2006. PMID: 16729300 - U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7.
Savarese E, Chae OW, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A. Savarese E, et al. Blood. 2006 Apr 15;107(8):3229-34. doi: 10.1182/blood-2005-07-2650. Epub 2005 Dec 20. Blood. 2006. PMID: 16368889 - C-reactive protein inhibits plasmacytoid dendritic cell interferon responses to autoantibody immune complexes.
Mold C, Clos TW. Mold C, et al. Arthritis Rheum. 2013 Jul;65(7):1891-901. doi: 10.1002/art.37968. Arthritis Rheum. 2013. PMID: 23576062 Free PMC article. - Emerging roles of TLR7 and TLR9 in murine SLE.
Santiago-Raber ML, Baudino L, Izui S. Santiago-Raber ML, et al. J Autoimmun. 2009 Nov-Dec;33(3-4):231-8. doi: 10.1016/j.jaut.2009.10.001. Epub 2009 Oct 21. J Autoimmun. 2009. PMID: 19846276 Review. - The role of innate immunity in the induction of autoimmunity.
Pisetsky DS. Pisetsky DS. Autoimmun Rev. 2008 Oct;8(1):69-72. doi: 10.1016/j.autrev.2008.07.028. Epub 2008 Aug 15. Autoimmun Rev. 2008. PMID: 18708168 Review.
Cited by
- COVID-19: A Review on Diagnosis, Treatment, and Prophylaxis.
Fierabracci A, Arena A, Rossi P. Fierabracci A, et al. Int J Mol Sci. 2020 Jul 21;21(14):5145. doi: 10.3390/ijms21145145. Int J Mol Sci. 2020. PMID: 32708112 Free PMC article. Review. - Guanine-modified inhibitory oligonucleotides efficiently impair TLR7- and TLR9-mediated immune responses of human immune cells.
Römmler F, Hammel M, Waldhuber A, Müller T, Jurk M, Uhlmann E, Wagner H, Vollmer J, Miethke T. Römmler F, et al. PLoS One. 2015 Feb 19;10(2):e0116703. doi: 10.1371/journal.pone.0116703. eCollection 2015. PLoS One. 2015. PMID: 25695778 Free PMC article. - COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression.
Zhou D, Dai SM, Tong Q. Zhou D, et al. J Antimicrob Chemother. 2020 Jul 1;75(7):1667-1670. doi: 10.1093/jac/dkaa114. J Antimicrob Chemother. 2020. PMID: 32196083 Free PMC article. - Developments in the scientific understanding of lupus.
Ardoin SP, Pisetsky DS. Ardoin SP, et al. Arthritis Res Ther. 2008;10(5):218. doi: 10.1186/ar2488. Epub 2008 Oct 10. Arthritis Res Ther. 2008. PMID: 18947369 Free PMC article. Review. - Translating nucleic acid-sensing pathways into therapies.
Junt T, Barchet W. Junt T, et al. Nat Rev Immunol. 2015 Sep 15;15(9):529-44. doi: 10.1038/nri3875. Epub 2015 Aug 21. Nat Rev Immunol. 2015. PMID: 26292638 Review.
References
- Hall, J.C., L. Casciola-Rosen, and A. Rosen. 2004. Altered structure of autoantigens during apoptosis. Rheum. Dis. Clin. North Am. 30:455–471. - PubMed
- Amoura, Z., J.C. Piette, H. Chabre, P. Cacoub, T. Papo, B. Wechsler, J.F. Bach, and S. Koutouzov. 1997. Circulating plasma levels of nucleosomes in patients with systemic lupus erythematosus: correlation with serum antinucleosome antibody titers and absence of clear association with disease activity. Arthritis Rheum. 40:2217–2225. - PubMed
- Herrmann, M., R.E. Voll, O.M. Zoller, M. Hagenhofer, B.B. Ponner, and J.R. Kalden. 1998. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41:1241–1250. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous