How to build a central synapse: clues from cell culture - PubMed (original) (raw)
Review
How to build a central synapse: clues from cell culture
Ann Marie Craig et al. Trends Neurosci. 2006 Jan.
Abstract
Central neurons develop and maintain molecularly distinct synaptic specializations for excitatory and inhibitory transmitters, often only microns apart on their dendritic arbor. Progress towards understanding the molecular basis of synaptogenesis has come from several recent studies using a coculture system of non-neuronal cells expressing molecules that generate presynaptic or postsynaptic "hemi-synapses" on contacting neurons. Together with molecular properties of these protein families, such studies have yielded interesting clues to how glutamatergic and GABAergic synapses are assembled. Other clues come from heterochronic cultures, manipulations of activity in subsets of neurons in a network, and of course many in vivo studies. Taking into account these data, we consider here how basic parameters of synapses--competence, placement, composition, size and longevity--might be determined.
Figures
Figure 1
Molecular components of glutamatergic (a) and GABAergic (b) synapses. Only some of the components are shown, emphasizing cleft and transmembrane proteins and their interacting partners. Solid lines indicate reported protein–protein interactions; broken lines indicate presumed indirect interactions. For references, see main text.
Figure 2
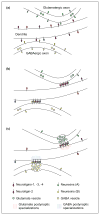
Hemi-synapse induction by neuroligins or neurexins presented to isolated axons or dendrites on the surface of fibroblasts. (a) Fibroblasts (F) expressing neuroligins induce clusters of presynaptic components, including GAD, at contact sites with axons of cultured neurons (N). These induced clusters of GAD (arrowheads) lack the normal postsynaptic proteins such as gephyrin, in contrast to endogenous synapses (arrow). (b) Fibroblasts (F) expressing neurexins induce clusters of postsynaptic components,including the GABAA receptor γ2 subunit (GABARγ2), at contact sites with dendrites of cultured neurons (N). These induced clusters of GABARγ2 (arrowheads) lack the normal presynaptic proteins such as synapsin, in contrast to endogenous synapses (arrow). (c) Hemi-presynapse (left) and hemi-postsynapse (right) formation in isolated axons and dendrites compared with bona fide synapses at axon–dendrite contacts (centre). Neuroligins, SynCAM, soluble FGF22 and soluble Wnt7a induce hemi-presynapses [18,21,29,55]; neurexins, NARP and ephrins induce full or partial hemi-postsynapses [60,67,71]. Scale bar, 10 μm.
Figure 3
Potential role for neurexins in matching postsynaptic and presynaptic composition. (a) It is possible that glutamatergic axons express neurexin isoforms (A) that bind most strongly to neuroligins-1, -3 and -4, whereas GABAergic axons express neurexins (B) that bind most strongly to neuroligin-2. Neurexins (A) versus (B) represent different forms that could result from differential gene usage, promoter usage, alternative splicing and/or glycosylation. Before synapse formation, all neuroligin isoforms might be diffusely distributed over the dendritic surface, whereas neurexins might be distributed ubiquitously over the axonal surface. (b) Synapse formation could be triggered when presynaptic neurexins interact with the appropriate postsynaptic neuroligins, stabilizing and aggregating both at nascent contact sites. (c) By specifically aggregating neuroligins-1, -3 and -4, glutamatergic neurexins could then cause the subsequent clustering of glutamate postsynaptic proteins. Likewise, by binding to and aggregating neuroligin-2, GABAergic neurexins could thereby influence the clustering of GABAergic postsynaptic proteins. In a complementary manner, the interaction of neuroligins with neurexins would also stabilize axon contacts and induce presynaptic specializations.
Figure 4
Comparison of predicted size differences between synaptic adhesion molecule pairs. The classical cadherin _trans_-dimer is modeled after an experimentally determined structure [108]. In this model, homophilic interaction between cadherin molecules located on opposing membrane surfaces is mediated by a strand exchange between the two extracellular cadherin 1 (EC1) domains. _Cis_-interactions (not shown) also contribute to defining the inter-membrane spacing of ~25 nm. The other structures are shown relative to a 20 nm inter-membrane spacing typical of a synaptic cleft [44]. The neurexin 1-β LNS domain structure is oriented so that the loops involved in interactions with neuroligins are oriented away from the membrane. Based on sequence homology, the crystal structure of acetylcholinesterase and the tandem fibronectin type III (FNIII) repeats of Drosophila neuroglian are used here to represent the size of the neuroligin ectodomain and the tandem EphB2 FNIII repeats, respectively. Ephrin-B2, EphB2, β-neurexins and neuroligins also have additional extracellular peptide sequence that cannot be modeled or predicted. Secondary structural elements are coded by color: red, α-helix; orange, 3–10 helix; green, β-sheet; yellow, turn; blue, coil. The structures were visualized using the Visual Molecular Dynamics (VMD) program [142] (
http://www.ks.uiuc.edu/Research/vmd/
). Protein Databank (PDB) files: C-cadherin (1L3W) [108], neurexin 1-β (1C4R) [143], mouse acetylcholinesterase (1MAA) [144], EphB2–Ephrin-B2 complex (1KGY) [145], Drosophila neuroglian (1CFB) [146].
Similar articles
- Balanced GABAergic and glutamatergic synapse development in hippocampal neurons.
Zhao X, Shoji S, Lau P. Zhao X, et al. Biochem Biophys Res Commun. 2005 May 20;330(4):1110-5. doi: 10.1016/j.bbrc.2005.03.083. Biochem Biophys Res Commun. 2005. PMID: 15823558 - Maturation of glutamatergic and GABAergic synapse composition in hippocampal neurons.
Anderson TR, Shah PA, Benson DL. Anderson TR, et al. Neuropharmacology. 2004 Oct;47(5):694-705. doi: 10.1016/j.neuropharm.2004.07.023. Neuropharmacology. 2004. PMID: 15458841 - Estimating transmitter release rates and quantal amplitudes in central synapses from postsynaptic current fluctuations.
Stepanyuk AR, Boychuk YA, Tsugorka TN, Drebot YI, Lushnikova IV, Pivneva TA, Belan PV. Stepanyuk AR, et al. Fiziol Zh (1994). 2004;50(4):22-32. Fiziol Zh (1994). 2004. PMID: 15460024 - Depolarization induced neuronal release of taurine in relation to synaptic transmission: comparison with GABA and glutamate.
Schousboe A, Sánchez Olea R, Pasantes-Morales H. Schousboe A, et al. Prog Clin Biol Res. 1990;351:289-97. Prog Clin Biol Res. 1990. PMID: 2173001 Review. No abstract available. - Enzymes involved in glutamatergic and GABAergic neurotransmission.
Kugler P. Kugler P. Int Rev Cytol. 1993;147:285-336. doi: 10.1016/s0074-7696(08)60771-8. Int Rev Cytol. 1993. PMID: 7901176 Review. No abstract available.
Cited by
- The role of neuronal complexes in human X-linked brain diseases.
Laumonnier F, Cuthbert PC, Grant SG. Laumonnier F, et al. Am J Hum Genet. 2007 Feb;80(2):205-20. doi: 10.1086/511441. Epub 2007 Jan 9. Am J Hum Genet. 2007. PMID: 17236127 Free PMC article. Review. - Excitation Control: Balancing PSD-95 Function at the Synapse.
Keith D, El-Husseini A. Keith D, et al. Front Mol Neurosci. 2008 Mar 28;1:4. doi: 10.3389/neuro.02.004.2008. eCollection 2008. Front Mol Neurosci. 2008. PMID: 18946537 Free PMC article. - Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization.
Takahashi H, Craig AM. Takahashi H, et al. Trends Neurosci. 2013 Sep;36(9):522-34. doi: 10.1016/j.tins.2013.06.002. Epub 2013 Jul 5. Trends Neurosci. 2013. PMID: 23835198 Free PMC article. Review. - Cochlear Synaptopathy and Noise-Induced Hidden Hearing Loss.
Shi L, Chang Y, Li X, Aiken S, Liu L, Wang J. Shi L, et al. Neural Plast. 2016;2016:6143164. doi: 10.1155/2016/6143164. Epub 2016 Sep 21. Neural Plast. 2016. PMID: 27738526 Free PMC article. Review. - Neurexin-neuroligin signaling in synapse development.
Craig AM, Kang Y. Craig AM, et al. Curr Opin Neurobiol. 2007 Feb;17(1):43-52. doi: 10.1016/j.conb.2007.01.011. Epub 2007 Feb 1. Curr Opin Neurobiol. 2007. PMID: 17275284 Free PMC article. Review.
References
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. - PubMed
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat Neurosci. 2001;4:569–578. - PubMed
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. - PubMed
- Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources