Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation - PubMed (original) (raw)
Comparative Study
Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation
Louis S Premkumar et al. J Neurosci. 2005.
Abstract
Transient receptor potential melastatin 8 (TRPM8) and transient receptor potential vanilloid 1 (TRPV1) are ion channels that detect cold and hot sensations, respectively. Their activation depolarizes the peripheral nerve terminals resulting in action potentials that propagate to brain via the spinal cord. These receptors also play a significant role in synaptic transmission between dorsal root ganglion (DRG) and dorsal horn (DH) neurons. Here, we show that TRPM8 is functionally downregulated by activation of protein kinase C (PKC) resulting in inhibition of membrane currents and increases in intracellular Ca2+ compared with upregulation of TRPV1 in cloned and native receptors. Bradykinin significantly downregulates TRPM8 via activation of PKC in DRG neurons. Activation of TRPM8 or TRPV1 at first sensory synapse between DRG and DH neurons leads to a robust increase in frequency of spontaneous/miniature EPSCs. PKC activation blunts TRPM8- and facilitates TRPV1-mediated synaptic transmission. Significantly, downregulation is attributable to PKC-mediated dephosphorylation of TRPM8 that could be reversed by phosphatase inhibitors. These findings suggest that inflammatory thermal hyperalgesia mediated by TRPV1 may be further aggravated by downregulation of TRPM8, because the latter could mediate the much needed cool/soothing sensation.
Figures
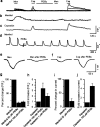
Figure 1.
Reciprocal modulation of TRPM8- and TRPV1-mediated currents by PKC in Xenopus oocytes expressing TRPM8 and TRPV1. a, Menthol-induced responses were reduced significantly by pretreatment with PDBu (1μ
m
; 5 min denoted by a break in the trace). b, Time course of PDBu-induced downregulation of TRPM8. c, Dose–response curve of PDBu-induced downregulation. d, Cold temperature (15–22°C)-induced currents were significantly reduced by PDBu. e, Not only is the amplitude of cold currents reduced, but the activation threshold is reduced also (shifted from 18.08 ± 0.15 to 17.12 ± 0.21°C; n = 4; p < 0.01) (dotted lines). f, Dose–response curves showing reduced sensitivity of the receptor after PDBu treatment; the EC50 value shifted from 56 to 156 μ
m
. g, I–V curve showing a ramp protocol that the TRPM8 current is rectifying and the block is seen at both positive and negative potentials before and after treatment with PDBu. h, Treatment with PKC inhibitor BIM reversed the reduction in current amplitude.i, In an oocyte injected with cRNA for both TRPM8 and TRPV1, the menthol-induced currents were significantly reduced (p < 0.001), whereas, capsaicin-induced currents were significantly potentiated (p < 0.05). j, Summary graph showing menthol- and cold-induced responses are significantly reduced (p<0.001) by PDBu or PMA, and the effect is reversed by the PKC inhibitor BIM. k, Summary graph showing capsaicin-induced currents are potentiated significantly (p < 0.01) and reversed by treatment with BIM. Cap, Capsaicin; Men, menthol. Data are represented as mean ± SEM.
Figure 2.
PKC activation blocks single-channel currents induced by menthol. Top, Single-channel currents activated by menthol at +60 mV from a cell-attached patch on an oocyte heterologously expressing TRPM8. Middle, Menthol-induced single-channel activity is reduced by incubation with PDBu for 5 min. Bottom, PDBu-induced reduction could be reversed after 5–10 min of washout. The right panel shows corresponding amplitude histograms, and the traces are shown at a higher time resolution below.
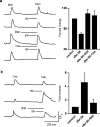
Figure 3.
Menthol- and capsaicin-induced increases in intracellular Ca2+ levels and membrane currents in DRG neurons were reduced and enhanced, respectively, by PDBu (1 μ
m
). a, Twenty selected neurons in a coverslip were individually tracked for changes in intracellular Ca2+ levels (F/_F_o) while exposing them to menthol (30μ
m
) and capsaicin (100 n
m
) before and after PDBu. Five neurons responded to menthol, two neurons responded to capsaicin, and one responded to both. Menthol responses were almost completely abolished(b), whereas capsaicin responses were significantly enhanced after PDBu(c). Note that capsaicin responses are larger and longer lasting. d, Time course of PDBu induced downregulation of menthol (100μ
m
)-induced response. e, Menthol (100μ
m
)-induced membrane currents were significantly reduced. f, Capsaicin (100 n
m
)-induced currents were significantly potentiated after pretreatment with PDBu. g, h, Summary graphs showing menthol-induced calcium responses are significantly reduced (p < 0.01), and capsaicin responses are significantly potentiated (100 n
m
capsaicin, p < 0.01; 1μ
m
capsaicin, p < 0.05) after PDBu. i, j, Summary graph showing significant inhibition (p < 0.01) of menthol-induced and significant potentiation (p < 0.01) of capsaicin-induced currents after PDBu. Cap, Capsaicin; Men, menthol. Data are represented as mean ± SEM.
Figure 4.
Reciprocal modulation of TRPM8 and TRPV1 by BK.a, Exposure of dissociated adult DRG neurons to BK (5μ
m
; 2 min) significantly decreased Ca2+ influx induced by menthol (100 μ
m
)(p < 0.01). This response could be reversed by incubating the neurons with a specific PKC inhibitor, BIM (500 n
m
), or protein phosphatase 1 inhibitor, okadaic acid (20 n
m
). b, In similar experimental conditions, the capsaicin response is significantly(p<0.01) potentiated by BIM. Cap, Capsaicin; Men, menthol; Oka, okadaic acid. Data are represented as mean ± SEM.
Figure 5.
Modulation of menthol- and capsaicin-mediated synaptic transmission at the first sensory synapse after PKC stimulation. a, Menthol-induced increases in mEPSCs were reduced by pretreatment with PDBu (control, 13; menthol, 165; menthol after PDBu, 53). Synaptic currents are shown in higher time resolution below.b, Decrease in interevent interval indicating an increase in the frequency of synaptic events, which were significantly reduced after treatment with PDBu. c, The increase in frequency of events was not accompanied by an increase in amplitude. d, Summary graphs showing menthol-induced significant increase (p < 0.01) in mEPSC frequency is abolished (p < 0.01) after PDBu. e, In similar experimental conditions, capsaicin-induced increases in the frequency of mEPSCs were further enhanced by PDBu (control, 7; menthol, 40; menthol after PDBu, 147). The synaptic current traces are shown in higher time resolution below. f, After PDBu, the frequency increased significantly. g, There was no change in amplitude before or after PDBu. h, Summary graph showing capsaicin-induced increase (p < 0.01) in the frequency is further significantly enhanced (p < 0.01) by PDBu. Cap, Capsaicin; Men, menthol. Data are represented as mean ± SEM.
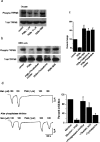
Figure 6.
Activation of PKC reduces TRPM8 phosphorylation. a, In oocytes, PDBu treatment dose dependently reduces phosphorylation (phospho TRPM8) of immunoprecipitated FLAG-TRPM8, which is reversed by pretreatment with BIM. b, HEK293T cells transiently transfected with FLAG-TRPM8 and treated with PDBu alone and in the presence of different PKC inhibitors, BIM (500 n
m
) and chelerythrine (20 μ
m
), or phosphatase inhibitor mixture show that PDBu-induced reduction in phosphorylated TRPM8 could be reversed by PKC and phosphatase inhibitors. c, Summary graph normalized to total TRPM8 levels showing significant decrease (p < 0.001) in phosphorylated TRPM8. PKC inhibitors (BIM, chelerythrine) and phosphatase inhibitors significantly reversed (p < 0.005, p < 0.005, and p < 0.005, respectively) the effect of PDBu and significantly increased basal phosphorylation. d, PMA-induced downregulation is reversed (p < 0.005) by pretreatment with phosphatase inhibitor mixture and okadaic acid (p < 0.05) but not by cyclosporine. Cap, Capsaicin. Data are represented as mean ± SEM.
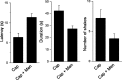
Figure 7.
Capsaicin-induced nocifensive behavior is reduced by coadministration with menthol. Latency, duration, and number of licks and/or shakes of the injected hindpaw were determined after intraplantar injection of 20μl of capsaicin (2 m
m
) or capsaicin plus menthol (5 m
m
). After coadministration of capsaicin plus menthol, the latency for licks and/or shakes was significantly increased from 6.39 ± 1 s (n = 19) to 11.4 ± 0.9 s (n = 23; p < 0.01). The duration of licks and/or shakes was also significantly decreased from 42.4 ± 4.5 to 27.3 ± 2.5 s (p < 0.01). Subsequently, we also counted the number of hindpaw licks and/or shakes with a cutoff time of 2 min. The number of characteristic hindpaw shakes was decreased significantly from 7.3 ± 1.4 to 3.8 ± 0.08 (p < 0.05). These findings suggest that there is a functional interaction between TRPM8 and TRPV1. Cap, Capsaicin; Men, menthol. Data are represented as mean ± SEM.
Similar articles
- Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse.
Sikand P, Premkumar LS. Sikand P, et al. J Physiol. 2007 Jun 1;581(Pt 2):631-47. doi: 10.1113/jphysiol.2006.118620. Epub 2007 Mar 15. J Physiol. 2007. PMID: 17363391 Free PMC article. - Roles of Thermosensitive Transient Receptor Channels TRPV1 and TRPM8 in Paclitaxel-Induced Peripheral Neuropathic Pain.
Li WW, Zhao Y, Liu HC, Liu J, Chan SO, Zhong YF, Zhang TY, Liu Y, Zhang W, Xia YQ, Chi XC, Xu J, Wang Y, Wang J. Li WW, et al. Int J Mol Sci. 2024 May 27;25(11):5813. doi: 10.3390/ijms25115813. Int J Mol Sci. 2024. PMID: 38892000 Free PMC article. - TRPM8 mechanism of cold allodynia after chronic nerve injury.
Xing H, Chen M, Ling J, Tan W, Gu JG. Xing H, et al. J Neurosci. 2007 Dec 12;27(50):13680-90. doi: 10.1523/JNEUROSCI.2203-07.2007. J Neurosci. 2007. PMID: 18077679 Free PMC article. - The cool things to know about TRPM8!
Iftinca M, Altier C. Iftinca M, et al. Channels (Austin). 2020 Dec;14(1):413-420. doi: 10.1080/19336950.2020.1841419. Channels (Austin). 2020. PMID: 33147416 Free PMC article. Review. - Regulation of TRPM8 channel activity.
Yudin Y, Rohacs T. Yudin Y, et al. Mol Cell Endocrinol. 2012 Apr 28;353(1-2):68-74. doi: 10.1016/j.mce.2011.10.023. Epub 2011 Oct 28. Mol Cell Endocrinol. 2012. PMID: 22061619 Free PMC article. Review.
Cited by
- The molecular and cellular basis of cold sensation.
McKemy DD. McKemy DD. ACS Chem Neurosci. 2013 Feb 20;4(2):238-47. doi: 10.1021/cn300193h. Epub 2012 Nov 28. ACS Chem Neurosci. 2013. PMID: 23421674 Free PMC article. Review. - Phospholipase C δ4 regulates cold sensitivity in mice.
Yudin Y, Lutz B, Tao YX, Rohacs T. Yudin Y, et al. J Physiol. 2016 Jul 1;594(13):3609-28. doi: 10.1113/JP272321. Epub 2016 May 29. J Physiol. 2016. PMID: 27062607 Free PMC article. - Topical hindpaw application of L-menthol decreases responsiveness to heat with biphasic effects on cold sensitivity of rat lumbar dorsal horn neurons.
Klein AH, Sawyer CM, Takechi K, Davoodi A, Ivanov MA, Carstens MI, Carstens E. Klein AH, et al. Neuroscience. 2012 Sep 6;219:234-42. doi: 10.1016/j.neuroscience.2012.05.061. Epub 2012 Jun 9. Neuroscience. 2012. PMID: 22687951 Free PMC article. - Dual regulation of TRPV1 by phosphoinositides.
Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Lukacs V, et al. J Neurosci. 2007 Jun 27;27(26):7070-80. doi: 10.1523/JNEUROSCI.1866-07.2007. J Neurosci. 2007. PMID: 17596456 Free PMC article. - Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals.
Madrid R, Donovan-Rodríguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Madrid R, et al. J Neurosci. 2006 Nov 29;26(48):12512-25. doi: 10.1523/JNEUROSCI.3752-06.2006. J Neurosci. 2006. PMID: 17135413 Free PMC article.
References
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW IV (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731. - PubMed
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860. - PubMed
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. - PubMed
- Cesare P, Dekker LV, Sardini A, Parker P, McNaughton PA (1999a) Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 23: 617–624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous