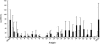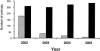Immunoglobulin G1 enzyme-linked immunosorbent assay for diagnosis of Johne's Disease in red deer (Cervus elaphus) - PubMed (original) (raw)
Immunoglobulin G1 enzyme-linked immunosorbent assay for diagnosis of Johne's Disease in red deer (Cervus elaphus)
J Frank T Griffin et al. Clin Diagn Lab Immunol. 2005 Dec.
Abstract
This study was designed to develop a customized enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Johne's disease (JD) in farmed deer. Two antigens were selected on the basis of their superior diagnostic readouts: denatured purified protein derivative (PPDj) and undenatured protoplasmic antigen (PpAg). ELISA development was based on the antigen reactivity of the immunoglobulin G1 (IgG1) isotype, which is a highly specific marker for mycobacterial disease seroreactivity in deer. Sensitivity estimates and test parameters were established using 102 Mycobacterium paratuberculosis-infected animals from more than 10 deer herds, and specificity estimates were determined using 508 uninfected animals from 5 known disease-free herds. A receiver-operated characteristic analysis determined that at a cut point of 50 ELISA units, there was a specificity of 99.5% and sensitivities of 84.0% with PPDj antigen, 88.0% with PpAg, and 91.0% when the antigens were used serially in a composite test. Estimated sensitivity was further improved using recombinant protein antigens unique for M. paratuberculosis, which identified infected animals that were unreactive to PPDj or PpAg. While 80% of animals that were seropositive in the IgG1 ELISA had detectable histopathology, the assay could also detect animals with subclinical disease. The test was significantly less sensitive (75%) for animals that were culture positive for M. paratuberculosis but with no detectable pathology than for those with pathological evidence of JD (>90%). When the IgG1 ELISA was used annually over a 4-year period in a deer herd with high levels of clinical JD, it eliminated clinical disease, increased production levels, and reduced JD-related mortality.
Figures
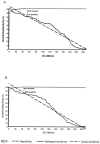
FIG. 1.
ROC curve analysis of IgG1 ELISA. Estimated sensitivity and specificity values using complex M. paratuberculosis protein antigens. Serum samples from 102 deer with confirmed M. paratuberculosis infection, as well as 508 samples obtained from deer herds with no prior history of JD, were assayed by IgG1 ELISA using PPDj (A) or PpAg (B) as the target antigen. Data were entered into a ROC analysis program; numerals refer to the calculated ELISA cut points and estimated test sensitivity values corresponding to 100% estimated test specificity for each antigen.
FIG. 2.
IgG1 antibody responses in _M. paratuberculosis_-infected and noninfected deer against a panel of recombinant M. paratuberculosis antigens. Sera from 10 _M. paratuberculosis_-infected deer (black bars) and 10 noninfected deer (white bars) were screened by IgG1 ELISA against a range of recombinant M. paratuberculosis antigens. Responses against complex protein antigens (PpAg and PPDj) are shown for comparison. Data represent mean EU ± standard errors of the mean.
FIG. 3.
Longitudinal changes in the proportion of test-positive and test-negative adult deer over a 4-year period following implementation of an IgG1-based ELISA test-and-cull management strategy. Regular serological screening for M. paratuberculosis infection was undertaken on a deer herd with a known history of JD. Screening commenced in 2002 (with an initial cut point of 100 EU) and proceeded over a 4-year period (with the cut point dropping to 50 EU after 6 months); test-positive animals were culled from the herd upon testing positive. Data represent longitudinal changes in the number of test-positive animals (gray bars) against test-negative animals (black bars) (data are shown for adult hinds only; data for stags not shown).
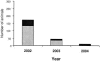
FIG. 4.
Longitudinal changes in the number of IgG1 ELISA-positive young deer following implementation of an ELISA-based test-and-cull management strategy. Young deer (fawns) were screened by IgG1 ELISA to detect M. paratuberculosis infection at two ages, 6 to 8 months (gray bars) and 12 to 15 months (black bars). Data represent the number of fawns testing positive at each time point, following the implementation of a test-and-cull management strategy in 2002.
Similar articles
- Absorbed EVELISA: a diagnostic test with improved specificity for Johne's disease in cattle.
Scott MC, Bannantine JP, Kaneko Y, Branscum AJ, Whitlock RH, Mori Y, Speer CA, Eda S. Scott MC, et al. Foodborne Pathog Dis. 2010 Nov;7(11):1291-6. doi: 10.1089/fpd.2010.0541. Epub 2010 Aug 12. Foodborne Pathog Dis. 2010. PMID: 20704508 - New method of serological testing for Mycobacterium avium subsp. paratuberculosis (Johne's disease) by flow cytometry.
Eda S, Elliott B, Scott MC, Waters WR, Bannantine JP, Whitlock RH, Speer CA. Eda S, et al. Foodborne Pathog Dis. 2005 Fall;2(3):250-62. doi: 10.1089/fpd.2005.2.250. Foodborne Pathog Dis. 2005. PMID: 16156706 - A highly sensitive and subspecies-specific surface antigen enzyme- linked immunosorbent assay for diagnosis of Johne's disease.
Eda S, Bannantine JP, Waters WR, Mori Y, Whitlock RH, Scott MC, Speer CA. Eda S, et al. Clin Vaccine Immunol. 2006 Aug;13(8):837-44. doi: 10.1128/CVI.00148-06. Clin Vaccine Immunol. 2006. PMID: 16893982 Free PMC article. - Bacterial diseases of farmed deer and bison.
Mackintosh C, Haigh JC, Griffin F. Mackintosh C, et al. Rev Sci Tech. 2002 Aug;21(2):249-63. doi: 10.20506/rst.21.2.1341. Rev Sci Tech. 2002. PMID: 11974613 Review.
Cited by
- Epidemiology, diagnostics, and management of tuberculosis in domestic cattle and deer in New Zealand in the face of a wildlife reservoir.
Buddle BM, de Lisle GW, Griffin JF, Hutchings SA. Buddle BM, et al. N Z Vet J. 2015 Jun;63 Suppl 1(sup1):19-27. doi: 10.1080/00480169.2014.929518. Epub 2015 Feb 3. N Z Vet J. 2015. PMID: 24992203 Free PMC article. Review. - Isolation of high-affinity single-chain antibodies against Mycobacterium avium subsp. paratuberculosis surface proteins from sheep with Johne's disease.
Berger S, Hinz D, Bannantine JP, Griffin JF. Berger S, et al. Clin Vaccine Immunol. 2006 Sep;13(9):1022-9. doi: 10.1128/CVI.00163-06. Clin Vaccine Immunol. 2006. PMID: 16960114 Free PMC article. - Innate immune markers that distinguish red deer (Cervus elaphus) selected for resistant or susceptible genotypes for Johne's disease.
Dobson B, Liggett S, O'Brien R, Griffin JF. Dobson B, et al. Vet Res. 2013 Jan 24;44(1):5. doi: 10.1186/1297-9716-44-5. Vet Res. 2013. PMID: 23347398 Free PMC article. - The modification and evaluation of an ELISA test for the surveillance of Mycobacterium avium subsp. paratuberculosis infection in wild ruminants.
Pruvot M, Forde TL, Steele J, Kutz SJ, De Buck J, van der Meer F, Orsel K. Pruvot M, et al. BMC Vet Res. 2013 Jan 9;9:5. doi: 10.1186/1746-6148-9-5. BMC Vet Res. 2013. PMID: 23302439 Free PMC article.
References
- Bannantine, J. P., J. F. J. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. - PubMed
- Buddle, B. M. 2001. Vaccination of cattle against Mycobacterium bovis. Tuberculosis (Edinburgh) 81:125-132. - PubMed
- Chinn, N. D., C. R. Rodgers, S. Liggett, E. Spittle, and J. F. T. Griffin. 2002. An alternative IgG1 ELISA test for Tb diagnosis in deer. Proc. Deer Branch N. Z. Vet. Assoc. 19:77-80.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources