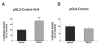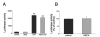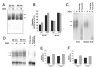Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism - PubMed (original) (raw)
Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism
Zoltán H Németh et al. J Immunol. 2005.
Abstract
Adenosine receptor ligands have anti-inflammatory effects and modulate immune responses by up-regulating IL-10 production by immunostimulated macrophages. The adenosine receptor family comprises G protein-coupled heptahelical transmembrane receptors classified into four types: A1, A2A, A2B, and A3. Our understanding of the signaling mechanisms leading to enhanced IL-10 production following adenosine receptor occupancy on macrophages is limited. In this study, we demonstrate that adenosine receptor occupancy increases IL-10 production by LPS-stimulated macrophages without affecting IL-10 promoter activity and IL-10 mRNA levels, indicating a posttranscriptional mechanism. Transfection experiments with reporter constructs containing sequences corresponding to the AU-rich 3'-untranslated region (UTR) of IL-10 mRNA confirmed that adenosine receptor activation acts by relieving the translational repressive effect of the IL-10 3'-UTR. By contrast, adenosine receptor activation failed to liberate the translational arrest conferred by the 3'-UTR of TNF-alpha mRNA. The IL-10 3'-UTR formed specific complexes with proteins present in cytoplasmic extracts of RAW 264.7 cells. Adenosine enhanced binding of proteins to a region of the IL-10 3'-UTR containing the GUAUUUAUU nonamer. The stimulatory effect of adenosine on IL-10 production was mediated through the A(2B) receptor, because the order of potency of selective agonists was 5'-N-ethylcarboxamidoadenosine (NECA) > N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide (IB-MECA) > 2-chloro-N6-cyclopentyladenosine (CCPA) = 2-p-(2-carboxyethyl)phenethylamino-5'-N-ethyl-carboxamidoadenosine (CGS-21680). Also, the selective A2B antagonist, alloxazine, prevented the effect of adenosine. Collectively, these studies identify a novel pathway in which activation of a G protein-coupled receptor augments translation of an anti-inflammatory gene.
Figures
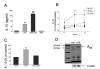
FIGURE 1
A, Adenosine (ADO) augments LPS-stimulated IL-10 production by LPS-activated RAW 264.7 macrophages. Adenosine (100 _μ_M) was added to cells immediately before immunostimulation with 10 _μ_g/ml LPS. IL-10 concentrations were measured from supernatants taken 5 h after stimulation with LPS. B, Effect of the A1 receptor agonist CCPA, A2A receptor agonist CGS-21680 (CGS), nonselective/A2B receptor agonist NECA, and A3 receptor agonist IB-MECA on LPS-induced IL-10 production by RAW cells. Selective agonists were added to cells immediately before immunostimulation with 10 _μ_g/ml LPS. IL-10 concentrations were measured from supernatants taken 5 h after stimulation with LPS. C, The A2B receptor antagonist alloxazine (ALLO) prevents the effect of adenosine on IL-10 production. Alloxazine (10 _μ_M) was administered 30 min before adenosine (100 _μ_M) and LPS (10 _μ_g/ml) followed by a 5-h-long incubation, after which supernatants were taken for IL-10 ELISA. D, Western blot analysis confirms expression of A2B receptors in membrane fractions from RAW 264.7 macrophages. LPS (10 _μ_g/ml) treatment of the cells for 3 h up-regulates A2B receptor expression. _β_-actin was used as control for protein loading. Results (mean ± SEM) shown are representative of at least three experiments with n = 4 in each experiment, with the exception of D, where n = 2 for each group. **, p < 0.01.
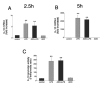
FIGURE 2
Lack of effect of adenosine (ADO, 100 _μ_M) on LPS (LPS, 10 _μ_g/ml)-induced IL-10 mRNA accumulation in RAW 264.7 cells. Adenosine was added to cells immediately before immunostimulation with LPS. IL-10 mRNA concentrations were measured by real-time PCR using RNA isolated 2.5 (A) or 5 h (B) after stimulation with LPS. C, Adenosine treatment (100 _μ_M) has no effect on LPS (10 _μ_g/ml)-induced IL-10 promoter activity in RAW 264.7 cells. To measure IL-10 promoter activity, cells were transiently transfected with an IL-10 promoter construct (harboring an SV40 promoter) and control _Renilla_-luciferase vector. Cells were treated with adenosine in the presence or absence of LPS for 8 h. Firefly luciferase reporter activities were normalized against Renilla luciferase activities, and IL-10 promoter activity was expressed as the Firefly:Renilla ratio. Data are mean ± SEM of n = 3 wells. Three experiments with similar results were performed. **, p < 0.01 indicates a significant increase in IL-10 mRNA levels or promoter activity following adenosine administration.
FIGURE 3
A, Nucleotide sequences of three potential regulatory AU-rich regions (AU1, AU2, and AU3) of the IL-10 3′-UTR (AU4). Highlighted are potential regulatory nonamer motifs. AU1–4 regions were inserted after the luciferase coding sequence of the pGL3-control reporter vector. B, Luciferase activities generated using plasmids containing AU1-AU4 are sharply decreased when compared with that generated by the pGL3-control reporter vector. Luciferase activity was measured from cells lysed 9 h after transfection with the various plasmids and normalized to protein content. C, Luciferase mRNA levels in cells transfected with plasmids containing AU1-AU4 are no different from that found in cells transfected with the pGL3-control reporter vector. Luciferase mRNA concentrations were measured using real-time PCR from cells lysed 9 h after transfection with the various plasmids. Representative data (mean ± SEM of n = 4 wells) are shown. Experiments were repeated at least three times. **, p < 0.01 vs pGL3-control.
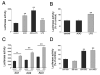
FIGURE 4
A, Effect of adenosine (ADO, 100 _μ_M) and/or LPS (LPS, 10 _μ_g/ml) on luciferase activity in RAW 264.7 cells transfected with a IL-10 luciferase reporter plasmid containing the entire 3′-UTR of IL-10 (AU4) downstream of the luciferase gene. Luciferase activity was measured from cells lysed 9 h after transfection with AU4 and normalized to protein content. ADO or LPS were administered 4 h after the transfection. B, Effect of ADO or LPS on luciferase activity in RAW 264.7 cells transfected with the pGL3-control luciferase vector. Luciferase activity was measured from cells lysed 9 h after transfection and normalized to protein content. ADO or LPS were administered 4 h after the transfection. C, ADO augments luciferase activity in RAW 264.7 cells transfected with IL-10 luciferase reporter plasmids containing various regions of the 3′-UTR of IL-10 (AU1–3) downstream of the luciferase gene. Luciferase activity was measured from cells lysed 9 h after transfection and normalized to protein content. ADO was administered 4 h after the transfection. D, The p38 MAPK inhibitor SB203580 (SB, 1 _μ_M) cotreatment fails to decrease the ADO-stimulated increase in AU4 luciferase activity. Results (mean ± SEM) shown are representative of at least three experiments with n = 4 in each experiment. **, p < 0.01; con, control.
FIGURE 5
A, The selective A2B receptor agonist NECA (10 _μ_M) increases luciferase activity in RAW 264.7 cells transfected with an IL-10 luciferase reporter plasmid containing the entire 3′-UTR of IL-10 (AU4) downstream of the luciferase gene. Luciferase activity was measured from cells lysed 9 h after transfection with AU4 and normalized to protein content. NECA was administered 4 h after the transfection. B, NECA does not influence luciferase activity in RAW 264.7 cells transfected with the pGL3-control luciferase vector. Luciferase activity was measured from cells lysed 9 h after transfection and normalized to protein content. NECA was administered 4 h after the transfection. Results (mean ± SEM) shown are representative of at least three experiments with n = 4 in each experiment. ** p < 0.01.
FIGURE 6
A, Effect of adenosine (ADO, 10 _μ_M) and/or LPS (LPS, 10 _μ_g/ml) on luciferase activity in RAW 264.7 cells transfected with a posttranscriptional TNF-α luciferase reporter plasmid containing an insert corresponding to the entire 3′-UTR of the TNF-α mRNA. Luciferase activity was measured from cells lysed 9 h after transfection and normalized to protein content. ADO or LPS were administered 4 h after the transfection. B, NECA does not influence luciferase activity in RAW 264.7 cells transfected with the pGL3-control luciferase vector. Luciferase activity was measured from cells lysed 9 h after transfection and normalized to protein content. NECA was administered 4 h after the transfection. Results (mean ± SEM) shown are representative of at least three experiments with n = 4 in each experiment. **, p < 0.01.
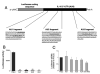
FIGURE 7
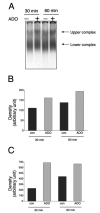
A, Adenosine (ADO) treatment of RAW 264.7 macrophages for 30 or 60 min increases RNA-protein complex formation between the AU2 region of the 3′-UTR of IL-10 mRNA and cellular components of RAW cells. Radiolabeled AU2 RNA probes were incubated with cytosolic fractions of RAW cells obtained at the end of the 30- or 60-min incubation period, complexes were separated by EMSA, and visualized using autoradiography. Two distinct complexes were observed in both untreated and treated cells. B, Densitometric analysis of intensities of the upper (■) and lower (
) complexes at 30 and 60 min following adenosine or control (con) treatment. C, Demonstration of the specificity of interactions between the AU2 region of the 3′-UTR of IL-10 mRNA and cellular fractions of RAW cells isolated 60 min after treatment with 100 μ_M adenosine. A 100× molar excess of cold AU2 oligonucleotide prevents formation of specific complexes in EMSA experiments (left panel). A mutated AU2 RNA probe lacking the GUAUUUAUU sequence forms a diffuse complex with cellular protein fractions of RAW cells as detected by EMSA (right panel). A 100_× molar excess of cold mutated AU2 oligonucleotide prevents formation of this complex (right panel). D, UV cross-linking of cytosolic extracts from RAW 264.7 macrophages to an AU2 RNA probe results in formation of two specific complexes. Densitometric analysis indicates that adenosine treatment promotes formation of both the upper (E) and lower (F) complexes when measured using extracts taken 30 and 60 min after adenosine treatment of cells. Shown are representative results of a single experiment of three experiments with similar results.
FIGURE 8
Adenosine treatment of RAW 264.7 macrophages for 30 or 60 min increases RNA-protein complex formation between the AU3 region of the 3′-UTR of IL-10 mRNA and cellular components of RAW cells. Radiolabeled AU2 RNA probes were incubated with cytosolic fractions of RAW cells obtained at the end of the 30- or 60-min incubation period, complexes were separated by EMSA, and visualized using autoradiography. Two distinct complexes were observed in both untreated and treated cells (A). Densitometric analysis indicates that adenosine treatment increases the intensity of both the upper (B) and lower (C) complexes when measured using extracts taken 30 and 60 min after adenosine treatment of cells. The results shown are from a single experiment (representative of three independent experiments with similar results).
Similar articles
- Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process.
Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, Haskó G. Koscsó B, et al. J Immunol. 2012 Jan 1;188(1):445-53. doi: 10.4049/jimmunol.1101224. Epub 2011 Nov 23. J Immunol. 2012. PMID: 22116830 Free PMC article. - Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists.
Szabó C, Scott GS, Virág L, Egnaczyk G, Salzman AL, Shanley TP, Haskó G. Szabó C, et al. Br J Pharmacol. 1998 Sep;125(2):379-87. doi: 10.1038/sj.bjp.0702040. Br J Pharmacol. 1998. PMID: 9786512 Free PMC article. - Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes.
Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Link AA, et al. J Immunol. 2000 Jan 1;164(1):436-42. doi: 10.4049/jimmunol.164.1.436. J Immunol. 2000. PMID: 10605040 - The role of the adenosinergic system in lung fibrosis.
Della Latta V, Cabiati M, Rocchiccioli S, Del Ry S, Morales MA. Della Latta V, et al. Pharmacol Res. 2013 Oct;76:182-9. doi: 10.1016/j.phrs.2013.08.004. Epub 2013 Aug 28. Pharmacol Res. 2013. PMID: 23994158 Review. - The adenosine a2b receptor: its role in inflammation.
Ham J, Rees DA. Ham J, et al. Endocr Metab Immune Disord Drug Targets. 2008 Dec;8(4):244-54. doi: 10.2174/187153008786848303. Endocr Metab Immune Disord Drug Targets. 2008. PMID: 19075778 Review.
Cited by
- Adenosine augments IL-10 production by microglial cells through an A2B adenosine receptor-mediated process.
Koscsó B, Csóka B, Selmeczy Z, Himer L, Pacher P, Virág L, Haskó G. Koscsó B, et al. J Immunol. 2012 Jan 1;188(1):445-53. doi: 10.4049/jimmunol.1101224. Epub 2011 Nov 23. J Immunol. 2012. PMID: 22116830 Free PMC article. - Adenosine A(2B) receptor deficiency promotes host defenses against gram-negative bacterial pneumonia.
Barletta KE, Cagnina RE, Burdick MD, Linden J, Mehrad B. Barletta KE, et al. Am J Respir Crit Care Med. 2012 Nov 15;186(10):1044-50. doi: 10.1164/rccm.201204-0622OC. Epub 2012 Sep 20. Am J Respir Crit Care Med. 2012. PMID: 22997203 Free PMC article. - The regulation of IL-10 production by immune cells.
Saraiva M, O'Garra A. Saraiva M, et al. Nat Rev Immunol. 2010 Mar;10(3):170-81. doi: 10.1038/nri2711. Epub 2010 Feb 15. Nat Rev Immunol. 2010. PMID: 20154735 Review. - Adenosine receptor activation ameliorates type 1 diabetes.
Németh ZH, Bleich D, Csóka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabó C, Cronstein BN, Haskó G. Németh ZH, et al. FASEB J. 2007 Aug;21(10):2379-88. doi: 10.1096/fj.07-8213com. Epub 2007 Apr 3. FASEB J. 2007. PMID: 17405852 Free PMC article. - Adenosine in the Thymus.
Köröskényi K, Joós G, Szondy Z. Köröskényi K, et al. Front Pharmacol. 2017 Dec 22;8:932. doi: 10.3389/fphar.2017.00932. eCollection 2017. Front Pharmacol. 2017. PMID: 29311934 Free PMC article. Review.
References
- Mosmann TR, Schumacher JH, Fiorentino DF, Leverah J, Moore KW, Bond MW. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990;145:2938–2945. - PubMed
- Barsig J, Kusters S, Vogt K, Volk HD, Tiegs G, Wendel A. Lipopolysaccharide-induced interleukin-10 in mice: role of endogenous tumor necrosis factor-α. Eur J Immunol. 1995;25:2888–2893. - PubMed
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-GM57982/GM/NIGMS NIH HHS/United States
- R01 AI031678-07/AI/NIAID NIH HHS/United States
- R01 GM66189/GM/NIGMS NIH HHS/United States
- R01 AI031678-06/AI/NIAID NIH HHS/United States
- R01 AI031678-08/AI/NIAID NIH HHS/United States
- R01 AI031678/AI/NIAID NIH HHS/United States
- R01 AI031678-12/AI/NIAID NIH HHS/United States
- R01 GM066189/GM/NIGMS NIH HHS/United States
- R01 AI031678-11/AI/NIAID NIH HHS/United States
- R03AR052038-01/AR/NIAMS NIH HHS/United States
- Z01 AA000375-02/Intramural NIH HHS/United States
- R01 GM066189-01/GM/NIGMS NIH HHS/United States
- R01 AI031678-09/AI/NIAID NIH HHS/United States
- R01 AI031678-10/AI/NIAID NIH HHS/United States
- R03 AR052038/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases