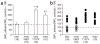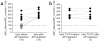CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels - PubMed (original) (raw)
CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels
Jianxi Liu et al. Nat Neurosci. 2006 Jan.
Abstract
High-conductance, Ca(2+)-activated and voltage-gated (BK) channels set neuronal firing. They are almost universally activated by alcohol, leading to reduced neuronal excitability and neuropeptide release and to motor intoxication. However, several BK channels are inhibited by alcohol, and most other voltage-gated K(+) channels are refractory to drug action. BK channels are homotetramers (encoded by Slo1) that possess a unique transmembrane segment (S0), leading to a cytosolic S0-S1 loop. We identified Thr107 of bovine slo (bslo) in this loop as a critical residue that determines BK channel responses to alcohol. In addition, the activity of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) in the cell controlled channel activity and alcohol modulation. Incremental CaMKII-mediated phosphorylation of Thr107 in the BK tetramer progressively increased channel activity and gradually switched the channel alcohol responses from robust activation to inhibition. Thus, CaMKII phosphorylation of slo Thr107 works as a 'molecular dimmer switch' that could mediate tolerance to alcohol, a form of neuronal plasticity.
Figures
Figure 1
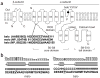
BK channels are made of pore-forming α-(slo) and modulatory β-subunits. (a) Depiction of a BK channel heterodimer showing its α- and β-subunits. Four slo subunits are thought to assemble together to make a functional BK channel. The diagram indicates the slo pore region (P), the domain that regulates the conductance of K+ (RCK domain), the Ca2+ bowl, and the core and tail domains, with the last two being joined by the linker. In the S0–S1 loop, the primary sequence alignment of key amino acids from bslo, mslo and hslo is shown; nonconserved residues are underlined. In the P region, the point mutation that introduces resistance to external TEA block is indicated. Accession numbers are given in parentheses. (b) Putative secondary structure of the S0–S1 loop and adjacent structures from mslo (left) and bslo (right). Columns represent the probability of finding a given secondary structure, as predicted by the PSIPRED server. C, coil; H, α-helix.
Figure 2
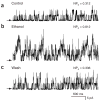
Ethanol at clinically relevant concentrations increases bslo T107V channel activity (NPo) after expression of these slo subunits in Xenopus oocytes. (a–c) Traces showing single channel recordings from the same I/O membrane patch obtained before (a), during (b) and 5 min after (c) exposure of the cytosolic side of the patch to 100 mM ethanol. Channel openings are shown as upward deflections; arrows indicate the baseline level. Channel NPo was obtained from all-points amplitude histograms, constructed from 60 s of continuous recording under each condition. The composition of the high [K+] solution facing both intracellular and extracellular sides of the patch is given in Methods. The membrane potential was set to 40 mV and the nominal free intracellular Ca2+ concentration was 300 nM.
Figure 3
The valine-to-threonine mutation in the slo S0–S1 loop significantly modifies BK channel responses to acute ethanol exposure. (a) Average changes in channel NPo in response to ethanol are shown as mean ± s.e.m. where n (number of patches or oocytes) is shown in parentheses at the bottom of each bar graph. In the presence of ethanol, NPo ratios reached 106.1 ± 15.6% (wild-type bslo), 123.6 ± 13.4% (mslo V86T), 247.8 ± 15.3% (bslo T107V) and 196.3 ± 16.7% (wild-type mslo). *P < 0.001 and #P < 0.01 in comparison to wild-type bslo; ¶P < 0.001 and ¶P < 0.05 in comparison to mslo V86T. The statistical difference between individual means was determined by one-way ANOVA followed by Bonferroni’s multiple-comparison test. (b) Acute exposure of the cytosolic side of I/O patches to 100 mM ethanol evoked varied responses in wild-type bslo and mslo V86T: activation (filled circles) or refractoriness/inhibition (hollow circles). In contrast, it consistently activated wild-type mslo and bslo T107V channels following subunit expression in the same batches of oocytes. The scatter graph depicts ratios of NPo values obtained in the presence and absence of ethanol (× 100); each data point represents an individual patch or oocyte. In both a and b, the dashed line indicates the point at which NPo is unchanged by ethanol.
Figure 4. In vitro treatment with alkaline phosphatase shifts wild-type bslo channels into a state that is readily activatable by ethanol, but does not modify bslo T107V channel responses to alcohol
(a) Before alkaline phosphatase (AP) treatment, acute exposure of the cytosolic side of I/O patches to 100 mM ethanol evoked varied responses in wild-type bslo: robust activation (filled circles) and refractoriness/inhibition (hollow circles). In contrast, after AP application (33 IU ml− 1 for 5 min) to the same patches, ethanol potentiated all wild-type bslo channels. (b) Ethanol responses of bslo T107V channels were identical before and after AP is applied to the same I/O membrane patches. In both a and b, the scatter graphs depict ratios of NPo values (× 100) obtained in the presence and absence of ethanol; each data point represents an individual patch or oocyte. Ethanol responses in a given patch before and after AP are connected by a dotted line. The dashed line indicates the point at which NPo is unchanged by alcohol.
Figure 5
In vitro treatment with KN-93, a selective CaMKII inhibitor, shifted wild-type bslo channels into a state that is readily activatable by ethanol, but it does not modify bslo T107V channel responses to alcohol. Acute exposure of the cytosolic side of I/O patches to 100 mM ethanol evoked varied responses from wild-type bslo channels: activation (filled circles) and refractoriness/inhibition (open circles). In sharp contrast, after KN-93 treatment (20 μM for 15 min), all wild-type bslo channels were activated by ethanol. Consistently, KN-92, an inactive analog of KN-93, failed to modify wild-type bslo channel responses. In contrast to wild-type bslo results, KN-93 treatment did not modify bslo T107V channel responses to ethanol when this subunit was expressed in the same batches of oocytes (compare the third column of data with the third column in Fig. 3b). The scatter graph depicts ratios of NPo values (× 100) obtained in the presence and absence of ethanol; each data point represents an individual patch or oocyte. The dashed line indicates the point at which NPo is unchanged by alcohol.
Figure 6
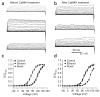
CaMKII increases wild-type bslo channel activity and switches channel responses to ethanol from activation to inhibition. (a,b) Current traces evoked at different potentials from excised I/O patches expressing wild-type bslo channels before (top), during (middle) and after (bottom) application of 100 mM ethanol to the cytosolic side of the patch. (a) Before CaMKII in vitro treatment, 100 mM ethanol reversibly increased wild-type bslo currents. (b) After CaMKII (1,000 IU ml− 1 for 5 min) was applied to the same patch, ethanol inhibited wild-type bslo currents (shift in _V_0.5 = − 80 mV; 200-ms depolarizing steps from − 40 mV to 160 mV, in 10-mV increments). Steady-state average current amplitude was determined 175–200 ms after the beginning of the depolarizing voltage step. (c,d) Averaged G/_G_max versus voltage data were fitted to a Boltzmann function. These fits show that both activated CaMKII and ethanol modified wild-type bslo currents by producing a parallel shift in the G/_G_max versus V relationship. The data (n = 5) were obtained under conditions and protocols identical to those used to evoke currents shown in a and b.
Figure 7
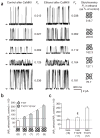
CaMKII phosphorylation of Thr107 progressively switches channel responses to ethanol. (a) Current traces from tetramers made of wild-type bslo and bslo T107V Y315V combinations recorded before and after ethanol administration, after CaMKII treatment. The top four traces show that as the proportion of T107V Y315V subunits decreased, so did the ethanol-induced potentiation (shown on the right of the traces as a percentage of control values). A cartoon depicts channel stoichiometry; the Y symbol indicates that Thr107 is phosphorylated. Tetramer composition was estimated from unitary currents, as Y315V introduces resistance to block by external TEA (Supplementary Note). The top four pairs of records were obtained in 2 mM external TEA. The fifth pair shows wild-type bslo currents in the absence of TEA. Recordings were obtained from I/O patches in symmetric 130 mM K+ with [Ca2+]ic ≈ 0.3 μM; V = +60 mV; arrows indicate the baseline. (b) The bar graph shows averaged ethanol responses. As the number of phosphorylated Thr107 residues increased, ethanol potentiation decreased, finally shifting to inhibition in phosphorylated bslo homotetramers. The white histogram bar indicates that wild-type bslo data were obtained in the absence of TEA. The dotted line shows linear regression fit to ethanol potentiation (r = 0.999). (c) T107E partially mimics the effect of CaMKII phosphorylation on bslo responses to ethanol. T107E homotetramers (second column in c) responded to ethanol as heterotetramers having two phosphorylated Thr107 (third column in b) (P > 0.05). ¶P < 0.001 in comparison to bslo T107V. Statistical differences between means were determined by ANOVA followed by Bonferroni’s test. In b and c, data are shown as mean ± s.e.m; the number under each histogram represents the number of patches or oocytes.
Similar articles
- Distinct sensitivity of slo1 channel proteins to ethanol.
Liu J, Bukiya AN, Kuntamallappanavar G, Singh AK, Dopico AM. Liu J, et al. Mol Pharmacol. 2013 Jan;83(1):235-44. doi: 10.1124/mol.112.081240. Epub 2012 Oct 23. Mol Pharmacol. 2013. PMID: 23093494 Free PMC article. - Functional effects of auxiliary beta4-subunit on rat large-conductance Ca(2+)-activated K(+) channel.
Ha TS, Heo MS, Park CS. Ha TS, et al. Biophys J. 2004 May;86(5):2871-82. doi: 10.1016/S0006-3495(04)74339-8. Biophys J. 2004. PMID: 15111404 Free PMC article. - Intrinsic electrostatic potential in the BK channel pore: role in determining single channel conductance and block.
Carvacho I, Gonzalez W, Torres YP, Brauchi S, Alvarez O, Gonzalez-Nilo FD, Latorre R. Carvacho I, et al. J Gen Physiol. 2008 Feb;131(2):147-61. doi: 10.1085/jgp.200709862. J Gen Physiol. 2008. PMID: 18227273 Free PMC article. - Ethanol interactions with calcium-dependent potassium channels.
Brodie MS, Scholz A, Weiger TM, Dopico AM. Brodie MS, et al. Alcohol Clin Exp Res. 2007 Oct;31(10):1625-32. doi: 10.1111/j.1530-0277.2007.00469.x. Alcohol Clin Exp Res. 2007. PMID: 17850640 Review. - Modulation of BK Channels by Ethanol.
Dopico AM, Bukiya AN, Kuntamallappanavar G, Liu J. Dopico AM, et al. Int Rev Neurobiol. 2016;128:239-79. doi: 10.1016/bs.irn.2016.03.019. Epub 2016 May 12. Int Rev Neurobiol. 2016. PMID: 27238266 Free PMC article. Review.
Cited by
- Modulation of KvAP unitary conductance and gating by 1-alkanols and other surface active agents.
Finol-Urdaneta RK, McArthur JR, Juranka PF, French RJ, Morris CE. Finol-Urdaneta RK, et al. Biophys J. 2010 Mar 3;98(5):762-72. doi: 10.1016/j.bpj.2009.10.053. Biophys J. 2010. PMID: 20197029 Free PMC article. - Calcium- and voltage-gated BK channels in vascular smooth muscle.
Dopico AM, Bukiya AN, Jaggar JH. Dopico AM, et al. Pflugers Arch. 2018 Sep;470(9):1271-1289. doi: 10.1007/s00424-018-2151-y. Epub 2018 May 11. Pflugers Arch. 2018. PMID: 29748711 Free PMC article. Review. - The epigenetic landscape of alcoholism.
Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. Krishnan HR, et al. Int Rev Neurobiol. 2014;115:75-116. doi: 10.1016/B978-0-12-801311-3.00003-2. Int Rev Neurobiol. 2014. PMID: 25131543 Free PMC article. Review. - Excitation of rat cerebellar Golgi cells by ethanol: further characterization of the mechanism.
Botta P, Simões de Souza FM, Sangrey T, De Schutter E, Valenzuela CF. Botta P, et al. Alcohol Clin Exp Res. 2012 Apr;36(4):616-24. doi: 10.1111/j.1530-0277.2011.01658.x. Epub 2011 Oct 17. Alcohol Clin Exp Res. 2012. PMID: 22004123 Free PMC article. - Acute alcohol action and desensitization of ligand-gated ion channels.
Dopico AM, Lovinger DM. Dopico AM, et al. Pharmacol Rev. 2009 Mar;61(1):98-114. doi: 10.1124/pr.108.000430. Epub 2009 Mar 6. Pharmacol Rev. 2009. PMID: 19270242 Free PMC article. Review.
References
- Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr. 1996;28:255–267. - PubMed
- Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K+ channel superfamily. News Physiol Sci. 1998;13:112–117. - PubMed
- Tian L, et al. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. - PubMed
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. - PubMed
- Krnjevic K. Excitable membranes. In: Fink BR, editor. Cellular Biology and Toxicity of Anesthetics. Williams and Wilkins; Baltimore: 1972. pp. 3–9.
Publication types
MeSH terms
Substances
Grants and funding
- R37 AA011560/AA/NIAAA NIH HHS/United States
- R01 AA011560-08/AA/NIAAA NIH HHS/United States
- AA11560/AA/NIAAA NIH HHS/United States
- R01 HL077424-02/HL/NHLBI NIH HHS/United States
- R01 AA011560/AA/NIAAA NIH HHS/United States
- R01 HL077424/HL/NHLBI NIH HHS/United States
- HL77424/HL/NHLBI NIH HHS/United States
- R29 AA011560/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous