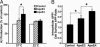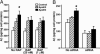Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target - PubMed (original) (raw)
Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target
Shiming Ye et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Apolipoprotein (apo) E4 is a major risk factor for Alzheimer's disease, and many studies have suggested that apoE has isoform-specific effects on the deposition or clearance of amyloid beta (Abeta) peptides. We examined the effects of apoE isoforms on the processing of amyloid precursor protein (APP) and on Abeta production in rat neuroblastoma B103 cells stably transfected with human wild-type APP695 (B103-APP). Lipid-poor apoE4 increased Abeta production in B103-APP cells to a greater extent than lipid-poor apoE3 (60% vs. 30%) due to more pronounced stimulation of APP recycling by apoE4 than apoE3. The difference in Abeta production was abolished by preincubating the cells with the receptor-associated protein (25 nM), which blocks the low-density lipoprotein receptor-related protein (LRP) pathway, or by reducing LRP expression by small interference RNA. The differences were also attenuated by replacing Arg-61 with threonine in apoE4 or pretreating apoE4 with small molecules, both of which abolish apoE4 intramolecular domain interaction. Thus, apoE4 appears to modulate APP processing and Abeta production through both the LRP pathway and domain interaction. These findings provide insights into why apoE4 is associated with increased risk for Alzheimer's disease and may represent a potential target for drug development.
Figures
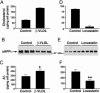
Fig. 1.
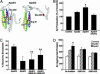
Effects of cellular cholesterol content and apoE isoforms on the secretion of sAPPα and Aβ. B103-APP cells were treated with β-VLDL (25 μg/ml cholesterol), lovastatin (4 μM), or medium alone (control), as described. Cellular cholesterol content was determined after treatment with β-VLDL (A) or lovastatin (D) treatment. sAPPα levels in 24-h conditioned medium were determined by using mAb 6E10 (1 μg/ml) after treatment with β-VLDL (B, lanes 1-3 and 4-6 are replicates of the same treatment) or lovastatin (E, lanes 1-4 and 5-8 are replicates of the same treatment). (C and F) Total Aβ in 24-h conditioned medium was detected by ELISA after treatment with β-VLDL (C) or lovastatin (F). Mean ± SD of two experiments, each repeated four to six times. *, P < 0.05 vs. control; **, P < 0.01 vs. control.
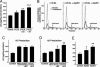
Fig. 2.
Lipid-poor apoE fractions or lipid-free apoE increase Aβ production in an isoform-specific manner. (A) ApoE3- or apoE4-enriched β-VLDL was prepared by incubating apoE isoforms with β-VLDL at 37°C for 1 h. Cells were then treated with either medium alone (control), β-VLDL (25 μg/ml cholesterol), or apoE-enriched β-VLDL (7.5 μg/ml apoE and 25 μg/ml cholesterol). Conditioned media were collected after 24 h and assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four times for each condition. (a) P < 0.05 vs. control; (b) P < 0.05 vs. β-VLDL; (c) P < 0.05 vs. β-VLDL + apoE3. (B) ApoE isoforms were incubated with β-VLDL at 37°C for 1 h. The apoE3- or apoE4-enriched β-VLDL and β-VLDL alone were then fractionated by fast-performance liquid chromatography as described. The elution profiles, which were monitored by quantitation of cholesterol and protein, showed two distinct fractions: a major β-VLDL or apoE-containing β-VLDL fraction and a smaller, lipid-poor apoE-containing fraction. (C and D) Samples from the major β-VLDL or apoE-containing β-VLDL fractions (C) were normalized by cholesterol content and incubated with B103-APP cells at 25 μg/ml cholesterol. Samples from the smaller, lipid-poor apoE-containing fractions (D) were normalized by protein content and incubated with the cells at 7.5 μg/ml protein. The 24-h conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four to six times for each condition. *, P < 0.05 vs. control (medium only). **, P < 0.05 vs. lipid-poor fraction of apoE3 or free apoE3. (E) Recombinant human apoE3 or apoE4 (7.5 μg/ml) was incubated with B103-APP cells for 24 h. The conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated four to six times for each condition. *, P < 0.05 vs. control (medium only). **, P < 0.05 vs. apoE3.
Fig. 3.
ApoE3 and apoE4 exert isoform-specific effects on Aβ production through their differential effects on intracellular APP recycling. (A) Blockage of APP recycling by culturing cells at low temperature abolished the apoE4-enhanced Aβ production. Recombinant human apoE3 or apoE4 (7.5 μg/ml) was incubated with B103-APP cells at either 22°C or 37°C for 24 h. The conditioned media were assayed for total Aβ by ELISA. Values are the mean ± SD of two experiments, each repeated four to six times for each condition. *, P < 0.05. (B) ApoE4 increased the internalization of cell-surface APP to a greater extent than apoE3. Internalization of cell-surface APP after apoE treatment was determined by measuring the uptake of radioiodinated 1G7 antibody, as described in Materials and Methods. The results are expressed as a ratio of the radioactivity associated with the internalized vs. cell-surface pools of APP. Values are the mean ± SD of two experiments, each repeated three times for each condition. *, P < 0.05.
Fig. 4.
The LRP mediates the enhancement of Aβ production by apoE4. (A) B103-APP cells were preincubated without or with RAP at a low concentration (25 nM), which blocks the LRP pathway, or a high concentration (1 μM), which blocks both the LRP and the LDL receptor pathway, at 37°C for 1 h and were then incubated with apoE3 or apoE4 (7.5 μg/ml) for 24 h. The conditioned media were assayed for total Aβ by ELISA. *, P < 0.05 vs. apoE3. (B) B103-APP cells were treated for 3 days with siRNA (2 μg of nucleotides per well) specific for the rat LRP gene and were then incubated with apoE3 or apoE4 (7.5 μg/ml) for 24 h. The conditioned media were collected 24 h after apoE treatment and assayed for total Aβ by ELISA. Values are the mean ± SD of percent of control B103 cells without apoE treatment (n = 4). *, P < 0.05 vs. apoE3.
Fig. 5.
ApoE4 domain interaction is responsible for the enhancement of Aβ production by apoE4. (A) A model of apoE4 domain interaction as a target for drug development. (B) B103-APP cells were incubated with apoE3, apoE4, or apoE4-Thr-61 (7.5 μg/ml) at 37°C for 24 h. The conditioned media were collected and assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated four times for each condition. *, P < 0.05 vs. apoE3 or apoE4-Thr-61. (C) Both GIND-25 (disulfonate) and GIND-105 (monosulfoalkyl) are capable of blocking apoE4 domain interaction as determined by a VLDL-like emulsion binding assay. Values are the mean ± SD of five to eight assays. **, P < 0.01 for both compounds vs. apoE4 alone. (D) Compounds GIND-25 and -105 abolish the enhancement of Aβ production by apoE4. Recombinant human apoE3 or apoE4 (7.5 μg/ml) was preincubated with or without GIND-25 or -105 (5 μM) at 37°C for 30 min and then further incubated with B103-APP cells for 24 h. The conditioned media were collected and assayed for total Aβ by ELISA. Values are the mean ± SD of three experiments, each repeated three to five times for each condition. *, P < 0.05 vs. apoE3.
Similar articles
- Astrocytes down-regulate neuronal beta-amyloid precursor protein expression and modify its processing in an apolipoprotein E isoform-specific manner.
Vincent B, Smith JD. Vincent B, et al. Eur J Neurosci. 2001 Jul;14(2):256-66. doi: 10.1046/j.0953-816x.2001.01643.x. Eur J Neurosci. 2001. PMID: 11553277 - Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer's amyloid beta peptides.
Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, Frangione B, Ghiso J. Tokuda T, et al. Biochem J. 2000 Jun 1;348 Pt 2(Pt 2):359-65. Biochem J. 2000. PMID: 10816430 Free PMC article. - Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation.
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Buttini M, et al. J Neurosci. 2002 Dec 15;22(24):10539-48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. J Neurosci. 2002. PMID: 12486146 Free PMC article. - Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.
Butterfield DA, Mattson MP. Butterfield DA, et al. Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review. - Apolipoprotein E isoform-dependent effects on the processing of Alzheimer's amyloid-β.
Chai AB, Lam HHJ, Kockx M, Gelissen IC. Chai AB, et al. Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Sep;1866(9):158980. doi: 10.1016/j.bbalip.2021.158980. Epub 2021 May 24. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 34044125 Review.
Cited by
- Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function.
Zhong N, Ramaswamy G, Weisgraber KH. Zhong N, et al. J Biol Chem. 2009 Oct 2;284(40):27273-80. doi: 10.1074/jbc.M109.014464. Epub 2009 Aug 7. J Biol Chem. 2009. PMID: 19666463 Free PMC article. - Cross interactions between Apolipoprotein E and amyloid proteins in neurodegenerative diseases.
Loch RA, Wang H, Perálvarez-Marín A, Berger P, Nielsen H, Chroni A, Luo J. Loch RA, et al. Comput Struct Biotechnol J. 2023 Jan 20;21:1189-1204. doi: 10.1016/j.csbj.2023.01.022. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36817952 Free PMC article. Review. - Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation.
Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Manelli AM, et al. Neurobiol Aging. 2007 Aug;28(8):1139-47. doi: 10.1016/j.neurobiolaging.2006.05.024. Epub 2006 Jul 11. Neurobiol Aging. 2007. PMID: 16837105 Free PMC article. - Female mice with apolipoprotein E4 domain interaction demonstrated impairments in spatial learning and memory performance and disruption of hippocampal cyto-architecture.
Adeosun SO, Hou X, Shi L, Stockmeier CA, Zheng B, Raffai RL, Weisgraber KH, Mosley TH, Wang JM. Adeosun SO, et al. Neurobiol Learn Mem. 2019 May;161:106-114. doi: 10.1016/j.nlm.2019.03.012. Epub 2019 Apr 4. Neurobiol Learn Mem. 2019. PMID: 30954674 Free PMC article. - A Novel Apolipoprotein E Antagonist Functionally Blocks Apolipoprotein E Interaction With N-terminal Amyloid Precursor Protein, Reduces β-Amyloid-Associated Pathology, and Improves Cognition.
Sawmiller D, Habib A, Hou H, Mori T, Fan A, Tian J, Zeng J, Giunta B, Sanberg PR, Mattson MP, Tan J. Sawmiller D, et al. Biol Psychiatry. 2019 Aug 1;86(3):208-220. doi: 10.1016/j.biopsych.2019.04.026. Epub 2019 May 2. Biol Psychiatry. 2019. PMID: 31208706 Free PMC article.
References
- Mahley, R. W. (1988) Science 240, 622-630. - PubMed
- Mahley, R. W. & Rall, S. C., Jr. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C. R., Beaudet, A. L., Sly, W. S., Valle, D., Childs, B., Kinzler, K. W. & Vogelstein, B. (McGraw-Hill, New York), Vol. 2, pp. 2835-2862.
- Huang, Y. & Mahley, R. W. (1999) in Plasma Lipids and Their Role in Disease, eds. Barter, P. J. & Rye, K.-A. (Harwood, Amsterdam), pp. 257-284.
- Weisgraber, K. H., Roses, A. D. & Strittmatter, W. J. (1994) Curr. Opin. Lipidol. 5, 110-116. - PubMed
- Mahley, R. W. & Rall, S. C., Jr. (2000) Annu. Rev. Genomics Hum. Genet. 1, 507-537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS046465/NS/NINDS NIH HHS/United States
- HL37063/HL/NHLBI NIH HHS/United States
- R01 HL064162/HL/NHLBI NIH HHS/United States
- NS46465/NS/NINDS NIH HHS/United States
- R01 HL037063/HL/NHLBI NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- HL64162/HL/NHLBI NIH HHS/United States
- AG22074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous