Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation - PubMed (original) (raw)
Comparative Study
Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation
Ping Han et al. J Neurosci. 2005.
Abstract
Alzheimer's disease is cytopathologically characterized by loss of synapses and neurons, neuritic amyloid plaques consisting of beta-amyloid (Abeta) peptides, and neurofibrillary tangles consisting of hyperphosphorylated tau protein in susceptible brain regions. Abeta, which triggers a cascade of pathogenic events including tau phosphorylation and neuronal excitotoxicity, is proteolytically derived from beta-amyloid precursor protein (APP); the pathological and physiological functions of APP, however, remain undefined. Here we demonstrate that the level of tau phosphorylation in cells and brains deficient in APP is significantly higher than that in wild-type controls, resulting from activation of cyclin-dependent kinase 5 (CDK5) but not glycogen synthase kinase 3, the two major tau kinases. In addition, we show that overexpression of APP or its non-amyloidogenic homolog amyloid precursor-like protein 1 suppresses both basal and stress-induced CDK5 activation. The ectodomain of APP, sAPPalpha, is responsible for inhibiting CDK5 activation. Furthermore, neurons derived from APP-deficient mice exhibit reduced metabolism and survival rates and are more susceptible to excitotoxic glutamate-induced apoptosis. These neurons also manifest significant defects in neurite outgrowth compared with neurons from the wild-type littermates. The observed neuronal excitotoxicity/apoptosis is mediated through a mechanism involving CDK5 activation. Our study defines a novel neuroprotective function for APP in preventing tau hyperphosphorylation via suppressing overactivation of CDK5. We suggest that CDK5 activation, through a calcium/calpain/p25 pathway, plays a key role in neuronal excitotoxicity and represents an underlying mechanism for the physiological functions of APP.
Figures
Figure 1.
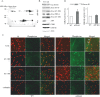
APP deficiency results in increased brain tau phosphorylation and CDK5 activity. Brain homogenates were prepared from APP KO and littermate control APP WT mice at 4 months of age. A, Two-dimensional PAGE analysis for determination of tau phosphorylation. One hundred micrograms of protein were used for the two-dimensional gel analysis. The first-dimension consisted of isoelectric focusing on a Protean IEF (Bio-Rad, Hercules, CA) (with a pH range of 3–10) cell, and the second-dimension consisted of SDS-PAGE separation using a precast criterion 4–15% gradient gel. B, Alteration of tau phosphorylation at various amino acid sites in APP KO brains. Brain homogenates were subjected to immunoprecipitation using H-150 antibody, which recognizes total tau protein, followed by Western blot analysis for total tau (using H-150 antibody) and for phosphorylated tau species using phospho-tau-specific antibodies AT-8, AT-100, PHF-1, and AT-180. Western blot assays for full-length APP (using antibody 369), CDK5, GSK3β, and actin were performed. C, Confocal microscopic study of different phospho-tau isoforms in the cortical regions of APP KO and APP WT mice. Frozen brain sections from wild-type mice (left) and APP KO mice (right) were probed with phospho-tau-specific antibodies AT-8, AT-100, AT-180, and trans-Golgi network marker protein γ-adaptin, followed by fluorescein-coupled secondary antibody (green). Cell nuclei were stained with propidium iodide (red). D, Tau kinase activity in APP KO and WT mouse brains. CDK5 activity was assayed by examining in vitro phosphorylation of histone-H1 protein as substrate in the presence of [γ-32P]ATP, followed by SDS-PAGE and autoradiography. GSK-3β activity was measured by in vitro phosphorylation of CREB peptide with GSK3β immunoprecipitates, followed by scintillation counting using a β-counter. Data represent mean ± SD; n = 4. *p < 0.005 versus WT control.
Figure 2.
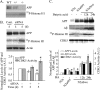
CDK5 activity inversely correlates with APP levels. A, CDK5 activity was measured in the brains of APP WT (+/+), heterozygous (+/–), and homozygous (–/–) KO mice. The levels of APP were measured by Western blot analysis. B, Downregulation of APP expression by siRNA induced CDK5 activity. N2a cells were transfected with APP siRNA or nonspecific/random siRNA [control (Cont.)] for 3 or 5 d. CDK5 activity was measured, and the levels of APP and actin were analyzed by Western blot. Actin was used for normalization of protein loading. Data represent mean ± SD; n = 3; *p < 0.01 compared with control siRNA. C, Distinct effects of APP and Aβ on CDK5 activity. Parental N2a cells, N2a cells stably expressing wild-type human APP 695, or the Swedish mutant form of APP 695 were incubated in the absence or presence of 5 m
m
butyric acid for 12 or 24 h, as indicated, to induce APP expression. APP, CDK5 activity, and CDK5 protein were assayed as described above. Aβ was determined by immunoprecipitation of cultured media with 4G8 antibody, followed by Western blotting using 6E10 antibody (Xu et al., 1998). Levels of APP, Aβ, and CDK5 activity were quantified and normalized to that of parental N2a cells [defined as 1 arbitrary unit (A.U.)]. Data represent mean ± SD; n = 3; *p < 0.05 compared with N2a parental cells.
Figure 3.
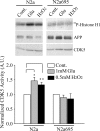
APP suppresses stress-mediated CDK5 activation. A, Parental N2a cells and N2a cells stably expressing WT APP695 were treated with stress-inducing agents, 1 m
m
glutamate, or 0.5 m
m
H2O2 for 5 h before being subjected to CDK5 kinase assays. B, CDK5 kinase activity was quantified and normalized to that of untreated controls. Data represent mean ± SD; n = 3. *p < 0.02, **p < 0.05 versus the control. Cont., Control, Glu, glutamate; A.U., arbitrary units.
Figure 4.
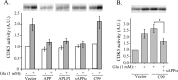
A, sAPPα and APLP1, but not APP βCTF, suppress excitotoxicity-induced CDK5 activation. CDK5 activity was assayed in N2a cells stably overexpressing vector alone, APP695, APLP1, sAPPα, or APP βCTF (C99) with or without previous treatment with 1 m
m
glutamate. CDK5 activities were quantified and normalized to that of N2a cells expressing control vector without glutamate treatment (defined as 1 arbitrary unit). Error bars indicate SD; n = 3. B, sAPPα is able to suppress glutamate-induced CDK5 activation in the presence of Aβ-bearing C99 fragment. A total of 10 n
m
purified sAPPα protein was added to N2a/APP βCTF (C99) cells pretreated with 1 m
m
glutamate, and CDK5 activity was assayed and quantified. Data represent means ± SD from three separate experiments; *p < 0.02. A.U., Arbitrary units.
Figure 5.
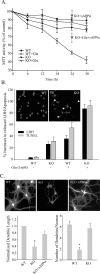
APP KO neurons exhibit reduced survival rates, increased susceptibility to apoptosis, and impaired neurite outgrowth. Primary cortical neurons were isolated from APP KO and littermate control APP WT mice at P0 and cultured for 2 weeks. A, Mitochondria function and survival of primary cortical neurons were assessed by MTT assay with and without 24 h glutamate (Glu) challenge. sAPPα (5 n
m
) was added to APP KO neurons from day 1 throughout the entire culture period (red symbols). Data represent mean ±SD; n = 3. B, Cell death in response to glutamate was assessed by released LDH, and apoptotic neurons were quantified by the percentage of MAP-2-positive pyknotic cells that colabeled with TUNEL and had condensed nuclei by DAPI staining 24 h after NMDA exposure. Two representative images are shown as insets: white arrowheads indicate apoptotic neurons. Numbers of neurons undergoing secondary necrosis (LDH) or apoptosis (TUNEL) were counted. Data represent mean ± SEM (n = 4). C, APP WT and KO neurons were grown on myelin-coated slides for 2 weeks, followed by immunostaining for MAP-2. The length of neurites was quantified from 50 MAP-2-positive cells, and the number of processes projecting from each positive cell was also quantified. Error bars indicate mean ± SD; p < 0.01 compared with WT controls, from three separate experiments.
Figure 6.
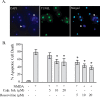
Calpain and CDK5 activation are involved in excitotoxicity of neurons. E17 rat primary hippocampal neuronal cultures were treated with 300 μ
m
NMDA for 15 min in the absence or presence of indicated concentrations of calpain inhibitors (Calp. Inh.) or CDK5 inhibitor (roscovitine). Images were captured 16 h after the treatment. A, Representative images of DAPI, TUNEL staining, and the merged picture of apoptotic neurons in response to NMDA exposure. The arrowhead indicates nonapoptotic nuclei. B, Apoptotic neurons are quantified and represented by the percentage of MAP-2-positive pyknotic cells that colabeled with TUNEL and had condensed nuclei on DAPI staining. Data are mean ± SEM (n = 4). *p < 0.05 versus NMDA alone.
Figure 7.
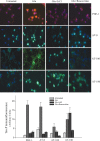
Excitotoxicity induces tau phosphorylation mediated by CDK5 activation. E17 rat hippocampal neurons were pretreated with 1 m
m
glutamate (Glu) with or without 5 m
m
LiCl or roscovitine for 30 min. Phospho-epitopes on tau proteins were examined by fluorescence microscopy using PHF1, AT-8, AT-100, or AT-180 antibodies. Representative micrographs of three separate experiments were presented (100× oil immersion objective). Immunofluorescence intensity of each tau-P epitope was quantified from four random fields (50 cells) and presented as fold increase over the negative control of secondary antibody alone.
Similar articles
- Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing.
Wen Y, Planel E, Herman M, Figueroa HY, Wang L, Liu L, Lau LF, Yu WH, Duff KE. Wen Y, et al. J Neurosci. 2008 Mar 5;28(10):2624-32. doi: 10.1523/JNEUROSCI.5245-07.2008. J Neurosci. 2008. PMID: 18322105 Free PMC article. - Deregulated Cdk5 activity is involved in inducing Alzheimer's disease.
Shukla V, Skuntz S, Pant HC. Shukla V, et al. Arch Med Res. 2012 Nov;43(8):655-62. doi: 10.1016/j.arcmed.2012.10.015. Epub 2012 Nov 7. Arch Med Res. 2012. PMID: 23142263 Free PMC article. Review. - Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation.
Sun LH, Ban T, Liu CD, Chen QX, Wang X, Yan ML, Hu XL, Su XL, Bao YN, Sun LL, Zhao LJ, Pei SC, Jiang XM, Zong DK, Ai J. Sun LH, et al. J Neurochem. 2015 Sep;134(6):1139-51. doi: 10.1111/jnc.13212. J Neurochem. 2015. PMID: 26118667 - Loss of Endothelial Nitric Oxide Synthase Promotes p25 Generation and Tau Phosphorylation in a Murine Model of Alzheimer's Disease.
Austin SA, Katusic ZS. Austin SA, et al. Circ Res. 2016 Oct 28;119(10):1128-1134. doi: 10.1161/CIRCRESAHA.116.309686. Epub 2016 Sep 6. Circ Res. 2016. PMID: 27601478 Free PMC article. - The molecular bases of Alzheimer's disease and other neurodegenerative disorders.
Maccioni RB, Muñoz JP, Barbeito L. Maccioni RB, et al. Arch Med Res. 2001 Sep-Oct;32(5):367-81. doi: 10.1016/s0188-4409(01)00316-2. Arch Med Res. 2001. PMID: 11578751 Review.
Cited by
- Hypothermia-induced hyperphosphorylation: a new model to study tau kinase inhibitors.
Bretteville A, Marcouiller F, Julien C, El Khoury NB, Petry FR, Poitras I, Mouginot D, Lévesque G, Hébert SS, Planel E. Bretteville A, et al. Sci Rep. 2012;2:480. doi: 10.1038/srep00480. Epub 2012 Jun 29. Sci Rep. 2012. PMID: 22761989 Free PMC article. - HIV and FIV glycoproteins increase cellular tau pathology via cGMP-dependent kinase II activation.
Sathler MF, Doolittle MJ, Cockrell JA, Nadalin IR, Hofmann F, VandeWoude S, Kim S. Sathler MF, et al. J Cell Sci. 2022 Jun 15;135(12):jcs259764. doi: 10.1242/jcs.259764. Epub 2022 Jun 21. J Cell Sci. 2022. PMID: 35638570 Free PMC article. - Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization.
Shirafuji N, Hamano T, Yen SH, Kanaan NM, Yoshida H, Hayashi K, Ikawa M, Yamamura O, Kuriyama M, Nakamoto Y. Shirafuji N, et al. Int J Mol Sci. 2018 Mar 17;19(3):891. doi: 10.3390/ijms19030891. Int J Mol Sci. 2018. PMID: 29562600 Free PMC article. - Holo-APP and G-protein-mediated signaling are required for sAPPα-induced activation of the Akt survival pathway.
Milosch N, Tanriöver G, Kundu A, Rami A, François JC, Baumkötter F, Weyer SW, Samanta A, Jäschke A, Brod F, Buchholz CJ, Kins S, Behl C, Müller UC, Kögel D. Milosch N, et al. Cell Death Dis. 2014 Aug 28;5(8):e1391. doi: 10.1038/cddis.2014.352. Cell Death Dis. 2014. PMID: 25165877 Free PMC article. - p35 and Rac1 underlie the neuroprotection and cognitive improvement induced by CDK5 silencing.
Posada-Duque RA, López-Tobón A, Piedrahita D, González-Billault C, Cardona-Gomez GP. Posada-Duque RA, et al. J Neurochem. 2015 Jul;134(2):354-70. doi: 10.1111/jnc.13127. Epub 2015 May 4. J Neurochem. 2015. PMID: 25864429 Free PMC article.
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA 97: 2910–2915. - PMC - PubMed
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15: 961–973. - PubMed
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med [Suppl] 10: S2–S9. - PubMed
- Buee L, Bussiere T, Buee-Scherrer V, Delacoutte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33: 95–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HD029587/HD/NICHD NIH HHS/United States
- F32 AG024895/AG/NIA NIH HHS/United States
- P01 HD29587/HD/NICHD NIH HHS/United States
- R01 AG024895/AG/NIA NIH HHS/United States
- R01 NS046673/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials