Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44 - PubMed (original) (raw)
Dentin matrix protein 1 enhances invasion potential of colon cancer cells by bridging matrix metalloproteinase-9 to integrins and CD44
Abdullah Karadag et al. Cancer Res. 2005.
Abstract
The up-regulation of various matrix metalloproteinases (MMP), certain cell receptors such as integrins and CD44, and the SIBLING family of integrin-binding glycophosphoproteins have been reported separately and in various combinations for many types of tumors. The mechanisms by which these different proteins may be interacting and enhancing the ability of a cancer cell to survive and metastasize have become an interesting issue in cancer biology. Dentin matrix protein 1 (DMP1) has been known for a number of years to bind to CD44 and ArgGlyAsp sequence-dependent integrins. This SIBLING was recently shown to be able to specifically bind and activate proMMP-9 and to make MMP-9 much less sensitive to inhibition by tissue inhibitors of metalloproteinases and synthetic inhibitors. In this study, we used a modified Boyden chamber assay to show that DMP1 enhanced the invasiveness of the MMP-9 expressing colon cancer cell line, SW480, through Matrigel in a dose-dependant manner. DMP1 (100 nmol/L) increased invasion 4-fold over controls (86.1 +/- 13.9 versus 22.3 +/- 9.8, P < 0.001). The enhanced invasive potential required the presence of MMP-9 and at least one of the cell surface receptors, CD44, alpha(v)beta(3), or alpha(v)beta(5) integrin. The bridging of MMP-9 to the cell surface receptors was shown by both pull-down and fluorescence activated cell sorting experiments. Because all of these proteins were also shown by immunohistochemistry to be expressed in serial sections of a colon adenocarcinoma, we have hypothesized that the MMP-9/DMP1/cell surface complexes observed to enhance cell invasion in vitro may be aiding metastatic events in vivo.
Figures
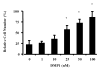
Fig. 1
Dentin matrix protein 1 (DMP1) and the invasion of the colon cancer cell line SW480 in vitro. Untreated or DMP1-treated SW480 colon cancer cells were placed in the top wells of separate Boyden chambers that each had Matrigel™-coated UV-opaque transwell inserts. The lower chambers contained serum-free conditioned medium as a chemoattractant. The cells were incubated at 37 °C for 24 h. Invasive cells that penetrated the Matrigel™ artificial basement membrane barrier and moved into the lower chamber were then detected by Calcein acetoxymethyl ester (AM) fluorescent dye activated by the live cells. The relative fluorescence (RF) in the lower chamber corresponded directly to the number of cells that digested their way through the barrier and migrated into to the lower chamber. Data are the means of triplicate samples, and error bars are the 95% confidence intervals. A P value of <0.001 was obtained for multiple comparisons within the panel by use of one-way analysis of variance. Each treatment group was also individually compared with the control (0 nM DMP1) group by use of the Dunnett test. *P<0.001, compared with untreated cells by the Dunnett test. All statistical tests were two-sided.
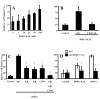
Fig. 2
RGD-dependent integrins and CD44 are involved in the DMP1-enhanced invasion of colon cancer cells in vitro. A) SW480 cells treated with a 0–100 nM DMP1-KAE (in which the RGD domain of DMP1 was replaced with the integrin-inactive tripeptide, KAE), were placed in the upper portion of a modified Boyden chamber coated with Matrigel™. The addition of DMP1-KAE resulted in a positive but less robust dose-response pattern compared to the integrin-binding native DMP1 (Fig. 1), showing that DMP1 does not have an absolute requirement to bind to RGD-dependent integrins to enhance invasion. B) Addition of CD44 antibodies (20 μg/ml) to the DMP1-KAE treatment completely negates the enhanced invasion showing that CD44 is the alternate cell surface-binding partner for DMP1. C) Blocking monoclonal antibodies to αvβ3 or αvβ5 integrins, or CD44 (20 μg/ml each antibody) were each able to partially block DMP1-enhanced invasion but not to control levels, suggesting that DMP1 can act through each of these cell surface receptors. Combined, the antibodies completely block the DMP1-enhanced invasion. Nonspecific IgGs had no affect on the invasion enhancement. D) Inhibition of MMP-9 activity by addition of blocking antibodies (20 μg/ml) returned both the DMP1- and DMP1-KAE-enhanced invasion to control levels. Relative fluorescence (RF), which corresponds to the number of cells that migrated through the Matrigel™, is as described in Fig. 1. Panel A data are the means of quadruplicate samples, and error bars are 95% confidence intervals. A P value of <0.01 was obtained for multiple comparisons within each panel, by use of one-way analysis of variance. Each treatment group was also individually compared with the control, untreated group by use of the Dunnett test. *P<0.01, compared with untreated cells by the Dunnett test. All statistical tests were two-sided. Data for panels B–D are the means of quadruplicate samples from a representative experiment, and error bars are 95% confidence intervals. A Mann-Whitney U test was used for the pairwise comparisons. A P value of <0.01 was obtained for SW480 cells treated DMP1-KAE or DMP1 vs. untreated respective control cells (*). A P value of <0.01 was obtained for SW480 cells treated with antibody+DMP−KAE (†) or DMP1 (‡) vs. isotype control IgG+DMP−KAE or DMP1, respectively. All statistical tests were two-sided.
Fig. 3
Pull-down experiments showing bridging of matrix metalloproteinase 9 (MMP-9) to αvβ3 integrin and αvβ5 integrin by dentin matrix protein 1 (DMP1). The αvβ3 or αvβ5 integrins were first bound to their respective monoclonal antibodies previously attached to beads by the manufacturer. After washing, the beads were incubated with buffer alone or buffer containing 500 nM DMP1-KAE or 500 nM DMP1, washed, and subsequently treated with recombinant proMMP-9. The washed samples were then electrophoresed on 10% zymogram gelatin gels and examined by Coomassie blue staining after digestion conditions were performed. Beads alone have low background level of MMP-9 binding (Lane 1). Note that the addition of DMP1 (Lane 4) but not DMP1-KAE (lane 3) enabled proMMP-9 to be pulled down with both sets of integrin-bound beads. Control levels of proMMP-9 were observed without addition of DMP1 (Lane 2).
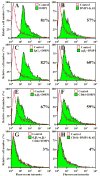
Fig. 4
Dentin matrix protein 1 (DMP1) enhanced binding of fluorescently labeled latent matrix metalloproteinase 9 (proMMP-9) to SW480 cells. ProMMP-9 was labeled with Alexa Fluor-488 and incubated with cells treated as indicated or left untreated cells (Control). Pretreating the cells with DMP1 (shaded area) increased the amount of labeled proMMP-9 bound to the living cells, compared with untreated cells as analyzed by FACS (A). Pretreating cells with DMP1-KAE (shaded area) showed increased binding of labeled proMMP-9, compared with that of untreated cells (open area) (B). Blocking cell-surface molecules αvβ3 integrin (D), αvβ5 integrin (E), or CD44 (F) with their respective monoclonal antibodies decreased the DMP1-enhanced binding of the labeled proMMP-9, but not to control levels. However, when all three antisera were added together (αvβ3 + αvβ5 + CD44) the DMP1-enhanced binding of labeled proMMP-9 was completely blocked (G). Treating cells with a nonimmune IgG had no affect on the ability of DMP1 to enhance the binding of proMMP-9 (C). DMP1-KAE-enhanced binding of labeled proMMP-9 was essentially blocked by CD44 monoclonal antibody alone (H). Numbers represent the percent of cells bound labeled proMMP-9.
Fig. 5
Co-localization of dentin matrix protein 1 (DMP1) and RGD-mutant DMP1 (DMP1-KAE) with matrix metalloproteinase 9 (MMP-9) on SW480 colon cancer cells. Cells were treated first with DMP1 and then with proMMP-9. The cells were incubated with 1) a mouse monoclonal antibody that can detect DMP1 as well as DMP1-KAE and 2) an affinity purified rabbit polyclonal antibody against MMP-9. Bound antibodies were then detected by indirect immunofluorescence with Cy2-conjugated AffiniPure goat anti-mouse IgG for DMP1 or DMP1-KAE, and Cy5-conjugated Affini-Pure goat anti-rabbit IgG for MMP-9. The green color in panel A shows the location of DMP1, the red color in panel B shows the location of MMP-9, and the yellow color in panel C shows that the two proteins co-localize. Similarly, the green color in panel D shows the location of DMP1-KAE, the red color in panel E shows the location of MMP-9, and the yellow color in panel F shows that the two proteins co-localize. 4, 6-Diamidino-2-phenylindole (DAPI) was used as a nuclear stain (blue color). Scale bar = 20 μm.
Fig. 6
The expression of dentin matrix protein 1 (DMP1), matrix metalloproteinase 9 (MMP-9), CD44, and all three subunits of integrin αvβ3 and αvβ5 in near-serial sections of moderately differentiated colon adenocarcinoma. Deparaffinized sections of a colon adenocarcinoma were incubated separately with antibodies specific for DMP1, MMP-9, CD44, and the αv, β3, and β5 integrin chains. Localization of the antibodies was determined with SuperPicture Polymer HRP-conjugated broad-spectrum secondary antibody and AEC Single Solution chromogen. Red/brown color indicates that DMP1 (Panel A), CD44 (Panel B), αv integrin (Panel C), β3 integrin(Panel D), β5 integrin(Panel E), MMP-9 (Panel F) are all associated with the same cells, suggesting that all proteins required for formation of complexes of CD44, or integrins αvβ3 or αvβ5, DMP1, and MMP-9 are expressed in the same cells. The negative controls: mouse IgG (Panel G), and rabbit immune serum (Panel H) revealed no signal. Mayer’s hematoxylin was used as a nuclear counterstain (purple-blue). Scale bar = 100 μm.
Similar articles
- Bone sialoprotein enhances migration of bone marrow stromal cells through matrices by bridging MMP-2 to alpha(v)beta3-integrin.
Karadag A, Fisher LW. Karadag A, et al. J Bone Miner Res. 2006 Oct;21(10):1627-36. doi: 10.1359/jbmr.060710. J Bone Miner Res. 2006. PMID: 16995818 - Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion.
Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Karadag A, et al. J Natl Cancer Inst. 2004 Jun 16;96(12):956-65. doi: 10.1093/jnci/djh169. J Natl Cancer Inst. 2004. PMID: 15199115 - A conjugate of camptothecin and a somatostatin analog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9.
Sun LC, Luo J, Mackey LV, Fuselier JA, Coy DH. Sun LC, et al. Cancer Lett. 2007 Feb 8;246(1-2):157-66. doi: 10.1016/j.canlet.2006.02.016. Epub 2006 Apr 27. Cancer Lett. 2007. PMID: 16644105 - Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer.
Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Bellahcène A, et al. Nat Rev Cancer. 2008 Mar;8(3):212-26. doi: 10.1038/nrc2345. Nat Rev Cancer. 2008. PMID: 18292776 Free PMC article. Review. - Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression.
Stefanidakis M, Koivunen E. Stefanidakis M, et al. Blood. 2006 Sep 1;108(5):1441-50. doi: 10.1182/blood-2006-02-005363. Epub 2006 Apr 11. Blood. 2006. PMID: 16609063 Review.
Cited by
- Single cell RNA sequencing analysis of mouse cochlear supporting cell transcriptomes with activated ERBB2 receptor indicates a cell-specific response that promotes CD44 activation.
Piekna-Przybylska D, Na D, Zhang J, Baker C, Ashton JM, White PM. Piekna-Przybylska D, et al. Front Cell Neurosci. 2023 Jan 6;16:1096872. doi: 10.3389/fncel.2022.1096872. eCollection 2022. Front Cell Neurosci. 2023. PMID: 36687526 Free PMC article. - Survey of dentin sialophosphoprotein and its cognate matrix metalloproteinase-20 in human cancers.
Aseervatham J, Geetu S, Anunobi CC, Koli K, Ogbureke KUE. Aseervatham J, et al. Cancer Med. 2019 May;8(5):2167-2178. doi: 10.1002/cam4.2117. Epub 2019 Apr 1. Cancer Med. 2019. PMID: 30932369 Free PMC article. - Adhesive and migratory effects of phosphophoryn are modulated by flanking peptides of the integrin binding motif.
Suzuki S, Kobuke S, Haruyama N, Hoshino H, Kulkarni AB, Nishimura F. Suzuki S, et al. PLoS One. 2014 Nov 14;9(11):e112490. doi: 10.1371/journal.pone.0112490. eCollection 2014. PLoS One. 2014. PMID: 25396425 Free PMC article. - The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled.
Rowe PS. Rowe PS. Cell Biochem Funct. 2012 Jul;30(5):355-75. doi: 10.1002/cbf.2841. Epub 2012 May 9. Cell Biochem Funct. 2012. PMID: 22573484 Free PMC article. Review. - Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1.
Ravindran S, Narayanan K, Eapen AS, Hao J, Ramachandran A, Blond S, George A. Ravindran S, et al. J Biol Chem. 2008 Oct 31;283(44):29658-70. doi: 10.1074/jbc.M800786200. Epub 2008 Aug 28. J Biol Chem. 2008. PMID: 18757373 Free PMC article.
References
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280(2):460–5. - PubMed
- Ogbureke KU, Fisher LW. Expression of SIBLINGs and Their Partner MMPs in Salivary Glands. J Dent Res. 2004;83(9):664–70. - PubMed
- Ogbureke KU, Fisher LW. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs) Kidney Int. 2005;68(1):155–66. - PubMed
- George A, Sabsay B, Simonian PA, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268(17):12624–30. - PubMed
- MacDougall M, Gu TT, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13(3):422–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous