Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells - PubMed (original) (raw)
Comparative Study
Comparison of gamma interferon-mediated antichlamydial defense mechanisms in human and mouse cells
Christine Roshick et al. Infect Immun. 2006 Jan.
Abstract
Gamma interferon (IFN-gamma)-induced effector mechanisms have potent antichlamydial activities that are critical to host defense. The most prominent and well-studied effectors are indoleamine dioxygenase (IDO) and nitric oxide (NO) synthase. The relative contributions of these mechanisms as inhibitors of chlamydial in vitro growth have been extensively studied using different host cells, induction mechanisms, and chlamydial strains with conflicting results. Here, we have undertaken a comparative analysis of cytokine- and lipopolysaccharide (LPS)-induced IDO and NO using an extensive assortment of human and murine host cells infected with human and murine chlamydial strains. Following cytokine (IFN-gamma or tumor necrosis factor alpha) and/or LPS treatment, the majority of human cell lines induced IDO but failed to produce NO. Conversely, the majority of mouse cell lines studied produced NO, not IDO. Induction of IDO in human cell lines inhibited growth of L2 and mouse pneumonitis agent, now referred to as Chlamydia muridarum MoPn equally in all but two lines, and inhibition was completely reversible by the addition of tryptophan. IFN-gamma treatment of mouse cell lines resulted in substantially greater reduction of L2 than MoPn growth. However, despite elevated NO production by murine cells, blockage of NO synthesis with the l-arginine analogue N-monomethyl-l-arginine only partially rescued chlamydial growth, suggesting the presence of another IFN-gamma-inducible antichlamydial mechanism unique to murine cells. Moreover, NO generated from the chemical nitric oxide donor sodium nitroprusside showed little direct effect on chlamydial infectivity or growth, indicating a natural resistance to NO. Finally, IFN-gamma-inducible IDO expression in human HeLa cells was inhibited following exogenous NO treatment, resulting in a permissive environment for chlamydial growth. In summary, cytokine- and LPS-inducible effectors produced by human and mouse cells differ and, importantly, these host-specific effector responses result in chlamydial strain-specific antimicrobial activities.
Figures
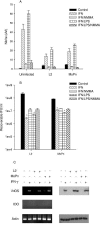
FIG. 1.
Effect of IFN-γ on the growth of C. trachomatis L2 and C. muridarum MoPn (A) and on the expression of iNOS and IDO (B) in HeLa cells. HeLa cells were untreated or pretreated with IFN-γ (20 U/ml) for 24 h, infected with chlamydiae, and then incubated in the complete culture medium described in Table 1. After 48 h of incubation, the cultures were harvested and chlamydial growth was assessed by determination of IFU following passing onto fresh HeLa monolayers. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. For RT-PCR and Western blot analyses, HeLa cells were untreated or pretreated with IFN-γ for 24 h and then left uninfected or infected with chlamydiae. Following incubation for a further 24 h, cells were harvested, RNA was isolated, cDNA was synthesized, and PCR was carried out using primers specific for human iNOS, IDO, and actin. For Western blot analyses, cells were harvested after 24 h and the cell pellets were resuspended in Laemmli sample buffer and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to nitrocellulose. See Materials and Methods for details.
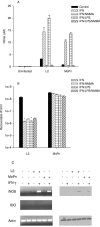
FIG. 2.
Effect of IFN-γ and IFN-γ-TNF-α-IL-1β-LPS on the growth of C. trachomatis L2 and C. muridarum MoPn (A and D) and on the expression of iNOS and IDO (B and C) in RT-4 cells. RT-4 cells were untreated or pretreated with IFN-γ (20 U/ml) for 24 h. Cells exposed to IFN-γ (20 U/ml)-TNF-α (20 ng/ml)-IL-1β (20 ng/ml)-LPS (20 ng/ml) were not pretreated. Following chlamydia infection, cells were incubated for a further 48 h in the complete medium described in Table 1 plus any indicated supplements. Final concentrations of tryptophan, indole, and NMMA were 500 μg/ml, 100 μM, and 1 mM, respectively. Cultures were harvested and processed for the various assays as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. Intracellular tryptophan pools were measured by HPLC. Data are presented as pmol tryptophan/107 cells and represent the means of two independent experiments with ranges shown. *, P < 0.0001 compared to the untreated control culture.
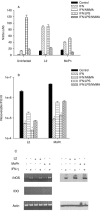
FIG. 3.
Effect of IFN-γ or IFN-γ-LPS on the growth of C. trachomatis L2 and C. muridarum MoPn (A) and on the expression of iNOS and IDO (B) in THP-1 monocyte cells. THP-1 cells were untreated or pretreated with IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) for 24 h, infected with chlamydiae, and then incubated in the complete culture medium described in Table 1. Cultures were harvested and processed for the various assays as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture.
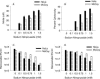
FIG. 4.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in McCoy cells. McCoy cells were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
FIG. 5.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in BM12.4 cells. BM12.4 cells were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
FIG. 6.
Effect of IFN-γ or IFN-γ-LPS on the expression of iNOS and IDO (A and C) and on the growth of C. trachomatis L2 and C. muridarum MoPn (B) in RAW 264.7 macrophages. RAW 264.7 macrophages were uninfected or infected with chlamydiae and then incubated in the complete culture medium described in Table 1 plus IFN-γ (20 U/ml) or IFN-γ (20 U/ml)-LPS (20 ng/ml) with or without NMMA (1 mM). Cultures were harvested and processed for the various assays 24 h later as described in the legend to Fig. 1. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
FIG. 7.
Effect of sodium nitroprusside-produced nitric oxide on HeLa and McCoy cells (A and B) and on the growth of C. trachomatis L2 (C) and C. muridarum (D) in HeLa and McCoy cells. HeLa and McCoy cells were infected with chlamydiae and then cultured in the appropriate complete medium (Table 1) plus the indicated concentration of SNP. After culturing for 48 h, medium nitrite levels were determined using the Griess reagent, host cell cytotoxicity was measured by assaying medium lactate dehydrogenase activity, and chlamydia growth was assessed by titrating recoverable IFU. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. See the legend to Fig. 1 and Materials and Methods for details. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite and represent the means of two independent experiments with ranges shown.
FIG. 8.
Effect of SNP-produced nitric oxide on IDO activity and C. trachomatis L2 growth in HeLa cells. HeLa cells were untreated or pretreated with IFN-γ for 24 h, then infected with C. trachomatis L2, and then cultured in complete medium (Table 1) supplemented with IFN-γ (20 U/ml) and/or sodium nitroprusside (0.5 mM). Following incubation for 48 h, medium nitrite levels were determined using Griess reagent, intracellular tryptophan pools were measured by HPLC, chlamydiae growth was assessed by titrating recoverable IFU, and IDO protein was detected by Western blotting. Growth data are presented as IFU (log10) and represent the means ± standard errors of triplicate determinations. *, P < 0.0001 compared to the untreated control culture. See the legend to Fig. 1 and Materials and Methods for details. Medium nitrite levels were determined after 48 h of incubation using the Griess reagent. Data are presented as μM nitrite (A) or pmol tryptophan/107 cells (B and C) and represent the means of two independent experiments with ranges shown. Intracellular tryptophan pools were measured by HPLC.
Similar articles
- The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide.
Igietseme JU. Igietseme JU. Immunology. 1996 Jan;87(1):1-8. Immunology. 1996. PMID: 8666420 Free PMC article. - Indoleamine 2,3-Dioxygenase Cannot Inhibit Chlamydia trachomatis Growth in HL-60 Human Neutrophil Granulocytes.
Virok DP, Tömösi F, Keller-Pintér A, Szabó K, Bogdanov A, Poliska S, Rázga Z, Bruszel B, Cseh Z, Kókai D, Paróczai D, Endrész V, Janáky T, Burián K. Virok DP, et al. Front Immunol. 2021 Nov 8;12:717311. doi: 10.3389/fimmu.2021.717311. eCollection 2021. Front Immunol. 2021. PMID: 34819931 Free PMC article. - Indoleamine 2,3-Dioxygenase Activity in Chlamydia muridarum and Chlamydia pneumoniae Infected Mouse Lung Tissues.
Virok DP, Raffai T, Kókai D, Paróczai D, Bogdanov A, Veres G, Vécsei L, Poliska S, Tiszlavicz L, Somogyvári F, Endrész V, Burián K. Virok DP, et al. Front Cell Infect Microbiol. 2019 Jun 12;9:192. doi: 10.3389/fcimb.2019.00192. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31249813 Free PMC article. - IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism.
Däubener W, MacKenzie CR. Däubener W, et al. Adv Exp Med Biol. 1999;467:517-24. doi: 10.1007/978-1-4615-4709-9_64. Adv Exp Med Biol. 1999. PMID: 10721095 Review. - The role of IFN-gamma in the outcome of chlamydial infection.
Rottenberg ME, Gigliotti-Rothfuchs A, Wigzell H. Rottenberg ME, et al. Curr Opin Immunol. 2002 Aug;14(4):444-51. doi: 10.1016/s0952-7915(02)00361-8. Curr Opin Immunol. 2002. PMID: 12088678 Review.
Cited by
- Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins.
Al-Zeer MA, Al-Younes HM, Lauster D, Abu Lubad M, Meyer TF. Al-Zeer MA, et al. Autophagy. 2013 Jan;9(1):50-62. doi: 10.4161/auto.22482. Epub 2012 Oct 19. Autophagy. 2013. PMID: 23086406 Free PMC article. - Regulation of chlamydial infection by host autophagy and vacuolar ATPase-bearing organelles.
Yasir M, Pachikara ND, Bao X, Pan Z, Fan H. Yasir M, et al. Infect Immun. 2011 Oct;79(10):4019-28. doi: 10.1128/IAI.05308-11. Epub 2011 Aug 1. Infect Immun. 2011. PMID: 21807906 Free PMC article. - Diverse requirements for SRC-family tyrosine kinases distinguish chlamydial species.
Mital J, Hackstadt T. Mital J, et al. mBio. 2011 Mar 22;2(2):e00031-11. doi: 10.1128/mBio.00031-11. Print 2011. mBio. 2011. PMID: 21427287 Free PMC article. - Chlamydia persistence -- a tool to dissect chlamydia--host interactions.
Schoborg RV. Schoborg RV. Microbes Infect. 2011 Jul;13(7):649-62. doi: 10.1016/j.micinf.2011.03.004. Epub 2011 Mar 31. Microbes Infect. 2011. PMID: 21458583 Free PMC article. Review. - Human conjunctival transcriptome analysis reveals the prominence of innate defense in Chlamydia trachomatis infection.
Natividad A, Freeman TC, Jeffries D, Burton MJ, Mabey DC, Bailey RL, Holland MJ. Natividad A, et al. Infect Immun. 2010 Nov;78(11):4895-911. doi: 10.1128/IAI.00844-10. Epub 2010 Sep 7. Infect Immun. 2010. PMID: 20823212 Free PMC article.
References
- Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907-916. - PubMed
- Bogdan, C. 1997. Of microbes, macrophages and nitric oxide. Behring Inst. Mitt. 99:58-72. - PubMed
- Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 12:64-76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials