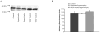Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis - PubMed (original) (raw)
Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis
I Friehs et al. Basic Res Cardiol. 2006 May.
Abstract
Objective: Pressure-overload hypertrophy is associated with decreased capillary density in myocardium resulting in impaired substrate delivery. Treatment of hypertrophied hearts with vascular endothelial growth factor (VEGF) induces angiogenesis. Since angiogenesis is associated with extracellular matrix degradation, we sought to determine whether VEGF induced angiogenesis in hypertrophy required matrix metalloproteinases (MMP) activation.
Methods: Newborn rabbits underwent aortic banding. Progression of hypertrophy (mass-to-volume (M/V) ratio) and mid-wall contractility index was monitored by echocardiography. At 4 and 6 weeks, VEGF (2 microg/kg), vehicle or VEGF combined with GM6001 (5 mg/kg), a MMP inhibitor, was administered intrapericardially. CD-31 (indicator of angiogenesis), MMP-2, MT1-MMP and TIMPs (endogenous MMP inhibitors) expression were measured by immunoblotting. MMP-2 activity was determined by gelatin zymography.
Results: Untreated hypertrophied hearts progressed to ventricular dilatation at 7 wks (M/V ratio: 0.75 +/- 0.07), but compensatory hypertrophy was maintained with VEGF (0.91 +/- 0.07; p < 0.05). LV contractility declined in untreated hearts from -0.41 +/- 0.9 (5 wks) to -0.73 +/- 0.5 (7 wks; p < 0.05) but remained normal with VEGF (+1.61 +/- 0.6 vs. +0.47 +/- 0.2). MMP-2 expression and activity were significantly elevated in VEGF treated hypertrophied hearts (p < 0.05) and were blocked by concomitant administration of GM6001. VEGF induced neovascularization was inhibited by addition of GM6001. MT1-MMP showed a trend to higher levels in VEGF treated hearts. TIMPs were unchanged in all three groups.
Conclusions: Exogenous VEGF and resultant MMP-2 activation leads to increased capillary formation in severe hypertrophy, preventing progression to ventricular dilation and dysfunction. VEGF and the associated MMP-2 activation play an important and potentially therapeutic role in vascular remodeling of hypertrophied hearts.
Figures
Fig. 1
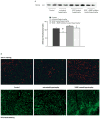
Microvascular density. A Representative immunoblots for CD-31 and summary of the densitometry data are depicted. VEGF treatment of hypertrophied hearts results in significant higher CD-31 levels. Concomitant administration of VEGF with a MMP inhibitor inhibited neoangiogenesis; however, the difference did not reach a level of significance (*p < 0.05 vs. VEGF treated hypertrophy). B Representative immunohistochemical sections of LV tissue are depicted with staining of the microvasculature with CD-31 in red or lectin in green
Fig. 2
Left ventricular mass to volume ratio and midwall contractility. A LV mass to LV cavity volume measurement as an indicator of hypertrophic growth. VEGF treated hearts maintained a higher ratio of LV mass to cavity volume over an extended period compared to untreated hypertrophied hearts, which showed signs of severe ventricular dilatation (= fall in M/V ratio) (*p < 0.05; versus untreated hypertrophied hearts). B Midwall contractility (depicted as Z-scores) was calculated based on echocardiographic measurements of non-hypertrophied control hearts. VEGF treatment prevented myocardial dysfunction seen in the untreated hypertrophied hearts (*p < 0.05; versus untreated hypertrophied hearts)
Fig. 3
MMP-2 activity. A, B MMP-2 activity levels were determined by gelatin zymography. Two representative gels are depicted with MMP activity for hypertrophied hearts and VEGF treated hearts at two different time points following treatment. Active MMP-2 was higher in VEGF treated hypertrophied hearts, one week following VEGF administration compared to untreated hypertrophied hearts. C A representative zymogram is shown which indicates that concomitant administration of VEGF and a MMP inhibitor decreased zymographic activity of MMP-2
Fig. 4
Myocardial MMP protein content. A To reconfirm that MMP-2 is activated within one week following VEGF treatment, we performed immunoblot analysis using an antibody identifying total and active MMP-2. A representative immunoblot for this specific MMP species is shown for immunoprecipitates obtained from left ventricular muscle extracts from controls, untreated hypertrophied hearts and VEGF treated hypertrophied hearts. A distinct immunoreactive band could be localized at 72 kDa indicative of the latent, non-active form and a second one at 66 kDa which represents the active form of MMP-2. B, C Quantification of protein content of latent (B) and active (C) form was performed by laser densitometry and values are expressed as arbitrary densitometry units. There is no difference of latent MMP-2 protein content between controls (solid bar) and VEGF treated hypertrophied hearts (shaded bar) and untreated hypertrophied hearts (blank bar) but active MMP-2 levels were significantly higher in hypertrophied hearts following VEGF treatment (*p < 0.05; versus untreated hypertrophy and Control)
Fig. 5
MT1-MMP protein content. AA representative immunoblot of MT1-MMP protein from left ventricular muscle extracts for all three groups is shown. BA summary of densitometry data showed that VEGF treatment resulted in an increase of MT1-MMP protein levels in hypertrophied hearts compared to untreated hearts but did not reach significance (p = 0.07)
Fig. 6
TIMP levels in myocardium. A–D Representative immunoblots for all four TIMPs and summary of the densitometry data are depicted. There was no significant difference in TIMP protein levels between the groups
Similar articles
- Vascular endothelial growth factor prevents apoptosis and preserves contractile function in hypertrophied infant heart.
Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ. Friehs I, et al. Circulation. 2006 Jul 4;114(1 Suppl):I290-5. doi: 10.1161/CIRCULATIONAHA.105.001289. Circulation. 2006. PMID: 16820588 Free PMC article. - Endogenous angiogenesis inhibitors prevent adaptive capillary growth in left ventricular pressure overload hypertrophy.
Nikolova A, Ablasser K, Wyler von Ballmoos MC, Poutias D, Kaza E, McGowan FX, Moses MA, Del Nido PJ, Friehs I. Nikolova A, et al. Ann Thorac Surg. 2012 Nov;94(5):1509-17. doi: 10.1016/j.athoracsur.2012.05.052. Epub 2012 Jul 12. Ann Thorac Surg. 2012. PMID: 22795062 Free PMC article. - Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females.
Voloshenyuk TG, Gardner JD. Voloshenyuk TG, et al. Am J Physiol Regul Integr Comp Physiol. 2010 Aug;299(2):R683-93. doi: 10.1152/ajpregu.00162.2010. Epub 2010 May 26. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 20504902 - Cardiac mast cell regulation of matrix metalloproteinase-related ventricular remodeling in chronic pressure or volume overload.
Janicki JS, Brower GL, Gardner JD, Forman MF, Stewart JA Jr, Murray DB, Chancey AL. Janicki JS, et al. Cardiovasc Res. 2006 Feb 15;69(3):657-65. doi: 10.1016/j.cardiores.2005.10.020. Epub 2005 Dec 22. Cardiovasc Res. 2006. PMID: 16376324 Review. - Matrix metalloproteinase inhibition and the prevention of heart failure.
Lee RT. Lee RT. Trends Cardiovasc Med. 2001 Jul;11(5):202-5. doi: 10.1016/s1050-1738(01)00113-x. Trends Cardiovasc Med. 2001. PMID: 11597832 Review.
Cited by
- Association of myocardial angiogenesis with structural and functional ventricular remodeling in aortic stenosis patients with normal ejection fraction.
Lee SP, Kim HK, Kim YJ, Oh S, Sohn DW. Lee SP, et al. J Cardiovasc Ultrasound. 2014 Jun;22(2):72-9. doi: 10.4250/jcu.2014.22.2.72. Epub 2014 Jun 30. J Cardiovasc Ultrasound. 2014. PMID: 25031797 Free PMC article. - The potential roles of exosomes in pathological cardiomyocyte hypertrophy mechanisms and therapy: A review.
Zhang L, Xie F, Zhang F, Lu B. Zhang L, et al. Medicine (Baltimore). 2024 Apr 26;103(17):e37994. doi: 10.1097/MD.0000000000037994. Medicine (Baltimore). 2024. PMID: 38669371 Free PMC article. Review. - Pressure overload induces early morphological changes in the heart.
Souders CA, Borg TK, Banerjee I, Baudino TA. Souders CA, et al. Am J Pathol. 2012 Oct;181(4):1226-35. doi: 10.1016/j.ajpath.2012.06.015. Epub 2012 Sep 4. Am J Pathol. 2012. PMID: 22954422 Free PMC article. - Role of mitochondrial fission and fusion in cardiomyocyte contractility.
Givvimani S, Pushpakumar SB, Metreveli N, Veeranki S, Kundu S, Tyagi SC. Givvimani S, et al. Int J Cardiol. 2015;187:325-33. doi: 10.1016/j.ijcard.2015.03.352. Epub 2015 Mar 25. Int J Cardiol. 2015. PMID: 25841124 Free PMC article. - Angiogenic Endothelial Cell Signaling in Cardiac Hypertrophy and Heart Failure.
Gogiraju R, Bochenek ML, Schäfer K. Gogiraju R, et al. Front Cardiovasc Med. 2019 Mar 6;6:20. doi: 10.3389/fcvm.2019.00020. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 30895179 Free PMC article. Review.
References
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89 (5):2183–2189. - PubMed
- Braunhut SJ, Moses MA. Retinoids modulate endothelial cell production of matrix-degrading proteases and tissue inhibitors of metalloproteinases (TIMP) J Biol Chem. 1994;269:13472–13479. - PubMed
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. - PubMed
- Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol. 1999;277:H777–H787. - PubMed
- Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous