The Bacillus subtilis spore coat provides "eat resistance" during phagocytic predation by the protozoan Tetrahymena thermophila - PubMed (original) (raw)
Comparative Study
. 2006 Jan 3;103(1):165-70.
doi: 10.1073/pnas.0507121102. Epub 2005 Dec 21.
Affiliations
- PMID: 16371471
- PMCID: PMC1324984
- DOI: 10.1073/pnas.0507121102
Comparative Study
The Bacillus subtilis spore coat provides "eat resistance" during phagocytic predation by the protozoan Tetrahymena thermophila
Lawrence A Klobutcher et al. Proc Natl Acad Sci U S A. 2006.
Abstract
Bacillus spores are highly resistant to many environmental stresses, owing in part to the presence of multiple "extracellular" layers. Although the role of some of these extracellular layers in resistance to particular stresses is known, the function of one of the outermost layers, the spore coat, is not completely understood. This study sought to determine whether the spore coat plays a role in resistance to predation by the ciliated protozoan Tetrahymena, which uses phagocytosis to ingest and degrade other microorganisms. Wild-type dormant spores of Bacillus subtilis were efficiently ingested by the protozoan Tetrahymena thermophila but were neither digested nor killed. However, spores with various coat defects were killed and digested, leaving only an outer shell termed a rind, and supporting the growth of Tetrahymena. A similar rind was generated when coat-defective spores were treated with lysozyme alone. The sensitivity of spores with different coat defects to predation by T. thermophila paralleled the spores' sensitivities to lysozyme. Spore killing by T. thermophila was by means of lytic enzymes within the protozoal phagosome, not by initial spore germination followed by killing. These findings suggest that a major function of the coat of spores of Bacillus species is to protect spores against predation. We also found that indigestible rinds were generated even from spores in which cross-linking of coat proteins was greatly reduced, implying the existence of a coat structure that is highly resistant to degradative enzymes.
Figures
Fig. 1.
Growth of Tetrahymena on B. subtilis spores. Plots of spore survival versus time (A) and Tetrahymena cells per ml versus time (B) are shown for wild-type spores (strain PS533) (open circles) and cotE spores (strain PS3394) (filled circles). Incubations were initiated with 2 × 103 Tetrahymena cells per ml and spores at an OD600nm = 7.5.
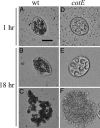
Fig. 2.
Coculture of Tetrahymena and B. subtilis spores. Tetrahymena cells fed wild-type (wt) (strain PS533) spores for 1 h (A) and 18 h (B) are shown. At both times, multiple dark bodies representing spore-filled phagosomes were seen within the Tetrahymena cells. Debris resembling the contents of phagosomes (C) is also evident at 18 h. Also shown are Tetrahymena fed cotE spores (strain PS3394) (cotE) for 1 h (D) or 18 h (E), and debris remaining from digested spores after 18 h (F). Images were obtained by brightfield microscopy at ×200 magnification. (Scale bar: 20 μm.)
Fig. 3.
Electron micrographs of Tetrahymena cells and spores. (A) Section of a Tetrahymena cell that had been coincubated with wild-type (strain PS533) spores for 20 h. Arrows indicate selected phagosomes or food vacuoles, and “ma” denotes the macronucleus. (B) Section of a Tetrahymena cell coincubated with cotE (strain PS3394) spores for 20 h. An early phagosome with partially digested spores (black arrow) and a late phagosome containing mostly spore rinds (white arrow) are indicated. (C) Extracellular wild-type spores after a 20-h coincubation with Tetrahymena. Note that intact spores were not completely permeable to fixatives but can be differentiated from spore rinds by the presence of internal, darkly staining material. (D) Extracellular rinds from cotE spores after a 20-h coincubation with Tetrahymena. A spore that appears to be germinating (arrowhead) and a vegetative bacterium (arrow) are also indicated. (E) Rinds produced by digestion of cotE spores with lysozyme. (Scale bars: 2 μm.)
Similar articles
- Protozoal digestion of coat-defective Bacillus subtilis spores produces "rinds" composed of insoluble coat protein.
Carroll AM, Plomp M, Malkin AJ, Setlow P. Carroll AM, et al. Appl Environ Microbiol. 2008 Oct;74(19):5875-81. doi: 10.1128/AEM.01228-08. Epub 2008 Aug 8. Appl Environ Microbiol. 2008. PMID: 18689521 Free PMC article. - Role of spore coat proteins in the resistance of Bacillus subtilis spores to Caenorhabditis elegans predation.
Laaberki MH, Dworkin J. Laaberki MH, et al. J Bacteriol. 2008 Sep;190(18):6197-203. doi: 10.1128/JB.00623-08. Epub 2008 Jun 27. J Bacteriol. 2008. PMID: 18586932 Free PMC article. - Transglutaminase-mediated cross-linking of GerQ in the coats of Bacillus subtilis spores.
Ragkousi K, Setlow P. Ragkousi K, et al. J Bacteriol. 2004 Sep;186(17):5567-75. doi: 10.1128/JB.186.17.5567-5575.2004. J Bacteriol. 2004. PMID: 15317760 Free PMC article. - [Identification and characterization of the outermost layer of Bacillus subtilis spores].
Imamura D. Imamura D. Yakugaku Zasshi. 2012;132(8):919-24. doi: 10.1248/yakushi.132.919. Yakugaku Zasshi. 2012. PMID: 22864350 Review. Japanese. - Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals.
Setlow P. Setlow P. J Appl Microbiol. 2006 Sep;101(3):514-25. doi: 10.1111/j.1365-2672.2005.02736.x. J Appl Microbiol. 2006. PMID: 16907802 Review.
Cited by
- Diverse supramolecular structures formed by self-assembling proteins of the Bacillus subtilis spore coat.
Jiang S, Wan Q, Krajcikova D, Tang J, Tzokov SB, Barak I, Bullough PA. Jiang S, et al. Mol Microbiol. 2015 Jul;97(2):347-59. doi: 10.1111/mmi.13030. Epub 2015 May 15. Mol Microbiol. 2015. PMID: 25872412 Free PMC article. - Role of dipicolinic acid in the germination, stability, and viability of spores of Bacillus subtilis.
Magge A, Granger AC, Wahome PG, Setlow B, Vepachedu VR, Loshon CA, Peng L, Chen D, Li YQ, Setlow P. Magge A, et al. J Bacteriol. 2008 Jul;190(14):4798-807. doi: 10.1128/JB.00477-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469099 Free PMC article. - Bacteria-phage coevolution with a seed bank.
Schwartz DA, Shoemaker WR, Măgălie A, Weitz JS, Lennon JT. Schwartz DA, et al. ISME J. 2023 Aug;17(8):1315-1325. doi: 10.1038/s41396-023-01449-2. Epub 2023 Jun 7. ISME J. 2023. PMID: 37286738 Free PMC article. - CotE binds to CotC and CotU and mediates their interaction during spore coat formation in Bacillus subtilis.
Isticato R, Pelosi A, De Felice M, Ricca E. Isticato R, et al. J Bacteriol. 2010 Feb;192(4):949-54. doi: 10.1128/JB.01408-09. Epub 2009 Dec 18. J Bacteriol. 2010. PMID: 20023017 Free PMC article. - Accumulation and Release of Rare Earth Ions by Spores of Bacillus Species and the Location of These Ions in Spores.
Dong W, Li S, Camilleri E, Korza G, Yankova M, King SM, Setlow P. Dong W, et al. Appl Environ Microbiol. 2019 Aug 14;85(17):e00956-19. doi: 10.1128/AEM.00956-19. Print 2019 Sep 1. Appl Environ Microbiol. 2019. PMID: 31253678 Free PMC article.
References
- Cano, R. J. & Borucki, M. (1995) Science 268, 1060–1064. - PubMed
- Kennedy, M. J., Reader, S. L. & Swierczynski, L. M. (1994) Microbiology 140, 2513–2529. - PubMed
- Vreeland, R. H., Rosenzweig, W. D. & Powers, D. W. (2000) Nature 407, 897–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases