Shiga toxin regulates its entry in a Syk-dependent manner - PubMed (original) (raw)
Shiga toxin regulates its entry in a Syk-dependent manner
Silje Ugland Lauvrak et al. Mol Biol Cell. 2006 Mar.
Abstract
Shiga toxin (Stx) is composed of an A-moiety that inhibits protein synthesis after translocation into the cytosol, and a B-moiety that binds to Gb3 at the cell surface and mediates endocytosis of the toxin. After endocytosis, Stx is transported retrogradely to the endoplasmic reticulum, and then the A-fragment enters the cytosol. In this study, we have investigated whether toxin-induced signaling is involved in its entry. Stx was found to activate Syk and induce rapid tyrosine phosphorylation of several proteins, one protein being clathrin heavy chain. Toxin-induced clathrin phosphorylation required Syk activity, and in cells overexpressing Syk, a complex containing clathrin and Syk could be demonstrated. Depletion of Syk by small interfering RNA, expression of a dominant negative Syk mutant (Syk KD), or treatment with the Syk inhibitor piceatannol inhibited not only Stx-induced clathrin phosphorylation but also endocytosis of the toxin. Also, Golgi transport of Stx was inhibited under all these conditions. In conclusion, our data suggest that Stx regulates its entry into target cells.
Figures
Figure 1.
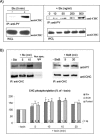
Tyrosine phosphorylation of clathrin in response to Stx. (A) HeLa cells were incubated with or without Stx (250 ng/ml) for 5 min (left) or with increasing concentrations of Stx for 5 min at 37°C (right). The cells were then lysed, and tyrosine phosphorylated proteins were immunoprecipitated by using the slurry of an anti-PY column. The column elution was analyzed by immunoblotting with mouse anti-CHC. As a control, a fraction of the WCL was run in parallel, and CHC was detected. (B) HeLa cells treated with or without Stx or StxB (250 ng/ml) for the indicated time points were lysed and immunoprecipitated with mouse anti-CHC antibody (X22). As a negative control, cells were also immunoprecipitated with nonspecific mouse IgG. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. To check equal loading, the membrane was then stripped and reprobed with anti-CHC. Note that all lanes shown for Stx are from the same blot, and all lanes shown for StxB are from the same blot. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC) after Stx and StxB treatment, and after pooling these results. The error bars show the SEM (n > 2) or the deviation (n = 2) for the different time points. Significant differences (p < 0.05) where determined by Student's t test and are indicated by a star; 0.05 < p <0.114 are indicated by open circles (○). Together, these p values indicate that the increase in CHC phosphorylation is significant.
Figure 2.
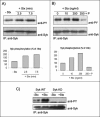
Autophosphorylation of Syk in response to Stx treatment. HeLa cells transfected with human Syk cDNA and treated with 250 ng/ml Stx for 2.5 or 7.5 min at 37°C (A) or with the indicated concentrations of Stx for 5 min after preincubation with and without piceatannol (P) (50 μM) for 30 min (B). The cells were then lysed, and the lysate was immunoprecipitated overnight with anti-Syk antibody. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. The membranes were then stripped and reprobed with mouse anti-Syk antibody. Graph, quantification of the amount of phosphorylated Syk (after normalization for the amount of immunoprecipitated Syk) in these experiments, which are representative experiments of at least three independent experiments. (C) HeLa cells transfected with cDNA encoding for Syk WT or Syk KD were incubated with and without 250 ng/ml Stx for 5 min at 37°C. Cell lysates were analyzed as described in A and B.
Figure 3.
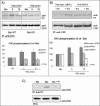
CHC phosphorylation results from Syk activation. (A) HeLa cells transfected with Syk WT or Syk KD were incubated with or without Stx for the different time points. The cells were lysed and clathrin was immunoprecipitated with anti-CHC antibodies overnight at 4°C. Finally, CHC tyrosine phosphorylation was monitored by Western blot analysis. The membrane was stripped and reprobed with anti-CHC to check equal loading of the material. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC). The error bars show the SEM (n = 3–4) or the deviation (n = 2) between the independent experiments. (B) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 were incubated with or without Stx (250 ng/ml) for 15 min. The cells were then lysed and immunoprecipitated as described in A. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC) from several experiments using both Syk siRNA 1 and Syk siRNA 2. The Syk mRNA level was reduced by 80–90 or 70–80% after transfection with siRNA 1 or 2, respectively. The error bars show the SEM (n = 4) or the deviation (n = 2) between the independent experiments. (C) Piceatannol inhibits tyrosine phosphorylation of clathrin. HeLa cells were pretreated with or without piceatannol (P) (50 μM) for 30 min and then incubated with 250 ng/ml Stx for 5 min at 37°C. The cells were then lysed, and proteins with phosphotyrosine were immunoprecipitated by using the slurry of an anti-phosphotyrosine (PY) column overnight at 4°C. The immunoprecipitates were analyzed by immunoblotting with mouse anti-CHC antibody. To show that equal amounts of cells were lysed and used for the immunoprecipitation, WCLs were loaded and analyzed.
Figure 4.
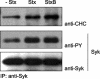
Coimmunoprecipitation of clathrin and Syk. HeLa cells were transfected with Syk WT and treated with 250 ng/ml Stx or Stx B-chain for 5 min at 37°C. The cells were then lysed and immunoprecipitated with anti-Syk antibodies immobilized on protein A/G beads overnight at 4°C. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. The membrane was then stripped and reprobed with mouse anti-Syk and then anti-CHC antibodies.
Figure 5.
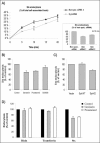
Syk is involved in Stx endocytosis. (A) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 or 2 were incubated with TAG- and biotin-labeled Stx (25 ng/ml) for the indicated time points. To remove the SS-linked biotin on the cell surface-bound toxin, half of the cells were then incubated with 0.1 M MESNa for 20 min on ice. Subsequently, the cells were washed and lysed, and the amount of TAG- and biotin-labeled toxin in the lysates was then measured using streptavidin beads and BioVeris detection system. Stx endocytosis was then plotted as percentage of total cell-associated toxin. Embedded graph, quantification of the degree of endocytosis (as percentage of nonspecific siRNA) after a 10-min incubation period. The Syk mRNA level was reduced by 80–90 or 70–80% after transfection with siRNA 1 or 2, respectively. The error bars show the deviation between two independent experiments, each done in triplicate. (B) HeLa cells preincubated with genistein (25 μg/ml), piceatannol (50 μM), or SU6656 (10 μM) for 30 min at 37°C were treated with TAG- and biotin-labeled Stx for 20 min and further treated as described in A. (C) HeLa cells transfected with the indicated constructs were incubated with TAG- and biotin-labeled Stx for 20 min and further treated as described in A. (D) Ricin and transferrin, both iodinated, were tested for uptake with the indicated inhibitors. Stx data were also plotted to facilitate a comparison. The error bars in A–C show the SEM between at least three independent experiments.
Figure 6.
Effect of genistein on clathrin-dependent and -independent Stx uptake. (A) BHK cells were induced (–tet)) or not (+tet) for the expression of antisense CHC for 2 d and tested in the presence or absence of genistein in a Stx uptake experiment carried out as described in the legend to Figure 5. The degree of endocytosis (as percentage of total cell-associated toxin) was then calculated. The error bars represent deviations between duplicates. The experiment shown is representative of eight independent experiments. (B) To verify that the clathrin-dependent endocytosis was blocked in the absence of tetracycline in our experiments, transferrin endocytosis was analyzed in parallel. Thus, cells were incubating with TAG- and biotin-labeled transferrin (50 ng/ml) for 5 min and further treated as described for Stx.
Figure 7.
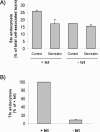
Effect of genistein and piceatannol on the sulfation of StxB-Sulf2. (A) HeLa cells were incubated with radioactive sulfate for 3 h at 37°C, and during the last 30 min, 50 μg/ml genistein or 50 μM piceatannol was added (duplicate samples). Then, StxB-Sulf2 (1 μg/ml) was added to the medium, and the incubation was continued for 1 h. The cells were subsequently washed, lysed, and immunoprecipitated with rabbit anti-Stx antibody overnight at 4°C. The adsorbed material was analyzed by 12% SDS-PAGE before autoradiography. The experiment shown is representative of at least three independent experiments. Graph, quantification of the amount of sulfated StxB. The error bars show the deviation between the two duplicates. (B) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 were incubated with radioactive sulfate and then StxB-Sulf2 as described in A. The Syk mRNA level was reduced by 80–90% after transfection with Syk siRNA 1. Graph, quantification of the amount of sulfated StxB. The error bars show the deviation between two independent experiments.
Figure 8.
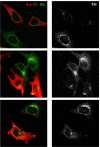
Syk KD is able to reduce Stx endocytosis and trafficking to the Golgi apparatus. HeLa cells were transfected 24 h before the experiment. They were then washed and incubated with Stx (250 ng/ml) for 45 min at 37°C. After fixation, the cells were visualized by confocal microscopy. Transfected cells were distinguished by using anti-Syk antibodies (red channel), and Stx was revealed by using anti-Stx antibodies (green channel). The transfected cells take up less Stx and also have less toxin in the Golgi area. In the panels to the right, Stx is shown in gray because this makes it easier to see the effect of the expression of Syk KD on its transport.
Similar articles
- Characterization of clathrin and Syk interaction upon Shiga toxin binding.
Wälchli S, Aasheim HC, Skånland SS, Spilsberg B, Torgersen ML, Rosendal KR, Sandvig K. Wälchli S, et al. Cell Signal. 2009 Jul;21(7):1161-8. doi: 10.1016/j.cellsig.2009.03.005. Epub 2009 Mar 13. Cell Signal. 2009. PMID: 19289168 - Shiga toxin increases formation of clathrin-coated pits through Syk kinase.
Utskarpen A, Massol R, van Deurs B, Lauvrak SU, Kirchhausen T, Sandvig K. Utskarpen A, et al. PLoS One. 2010 Jul 27;5(7):e10944. doi: 10.1371/journal.pone.0010944. PLoS One. 2010. PMID: 20668539 Free PMC article. - Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin.
Lauvrak SU, Torgersen ML, Sandvig K. Lauvrak SU, et al. J Cell Sci. 2004 May 1;117(Pt 11):2321-31. doi: 10.1242/jcs.01081. J Cell Sci. 2004. PMID: 15126632 - Endocytosis and retrograde transport of Shiga toxin.
Sandvig K, Bergan J, Dyve AB, Skotland T, Torgersen ML. Sandvig K, et al. Toxicon. 2010 Dec 15;56(7):1181-5. doi: 10.1016/j.toxicon.2009.11.021. Epub 2009 Nov 29. Toxicon. 2010. PMID: 19951719 Review. - Pathways followed by ricin and Shiga toxin into cells.
Sandvig K, Grimmer S, Lauvrak SU, Torgersen ML, Skretting G, van Deurs B, Iversen TG. Sandvig K, et al. Histochem Cell Biol. 2002 Feb;117(2):131-41. doi: 10.1007/s00418-001-0346-2. Epub 2001 Nov 20. Histochem Cell Biol. 2002. PMID: 11935289 Review.
Cited by
- The B subunits of Shiga-like toxins induce regulated VWF secretion in a phospholipase D1-dependent manner.
Huang J, Haberichter SL, Sadler JE. Huang J, et al. Blood. 2012 Aug 2;120(5):1143-9. doi: 10.1182/blood-2012-01-408096. Epub 2012 Jun 20. Blood. 2012. PMID: 22718838 Free PMC article. - Shiga toxin facilitates its retrograde transport by modifying microtubule dynamics.
Hehnly H, Sheff D, Stamnes M. Hehnly H, et al. Mol Biol Cell. 2006 Oct;17(10):4379-89. doi: 10.1091/mbc.e06-04-0310. Epub 2006 Aug 2. Mol Biol Cell. 2006. PMID: 16885418 Free PMC article. - Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis.
Day CA, Baetz NW, Copeland CA, Kraft LJ, Han B, Tiwari A, Drake KR, De Luca H, Chinnapen DJ, Davidson MW, Holmes RK, Jobling MG, Schroer TA, Lencer WI, Kenworthy AK. Day CA, et al. Traffic. 2015 Jun;16(6):572-90. doi: 10.1111/tra.12269. Epub 2015 Apr 27. Traffic. 2015. PMID: 25690058 Free PMC article. - Shiga toxin and its use in targeted cancer therapy and imaging.
Engedal N, Skotland T, Torgersen ML, Sandvig K. Engedal N, et al. Microb Biotechnol. 2011 Jan;4(1):32-46. doi: 10.1111/j.1751-7915.2010.00180.x. Microb Biotechnol. 2011. PMID: 21255370 Free PMC article. Review. - Lipid-mediated endocytosis.
Ewers H, Helenius A. Ewers H, et al. Cold Spring Harb Perspect Biol. 2011 Aug 1;3(8):a004721. doi: 10.1101/cshperspect.a004721. Cold Spring Harb Perspect Biol. 2011. PMID: 21576253 Free PMC article. Review.
References
- Bolen, J. B., and Brugge, J. S. (1997). Leukocyte protein tyrosine kinases: potential targets for drug discovery. Annu. Rev. Immunol. 15, 371–404. - PubMed
- Bonazzi, M., et al.. (2005). CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7, 570–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous