Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis - PubMed (original) (raw)
Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis
Thomas E Morrison et al. J Virol. 2006 Jan.
Abstract
Mosquito-borne alphaviruses are a significant cause of both encephalitic and arthritic disease in humans worldwide. In contrast to the encephalitic alphaviruses, the pathogenesis of alphavirus-induced arthritic disease is not well understood. Utilizing a mouse model of Ross River virus (RRV) disease, we found that the primary targets of RRV infection are bone, joint, and skeletal muscle tissues of the hind limbs in both outbred CD-1 mice and adult C57BL/6J mice. Moreover, histological analyses demonstrated that RRV infection resulted in severe inflammation of these tissues. Characterization of the inflammatory infiltrate within the skeletal muscle tissue identified inflammatory macrophages, NK cells, and CD4+ and CD8+ T lymphocytes. To determine the contribution of the adaptive immune system, the outcome of RRV-induced disease was examined in C57BL/6J RAG-1(-/-) mice, which lack functional T and B lymphocytes. RAG-1(-/-) and wild-type mice developed similar disease signs, infiltration of inflammatory macrophages and NK cells, and muscle pathology, suggesting that the adaptive immune response does not play a critical role in the development of disease. These results establish the mouse model of RRV disease as a useful system for the identification of viral and host factors that contribute to alphavirus-induced arthritis and myositis.
Figures
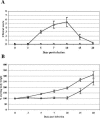
FIG. 1.
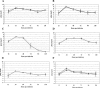
Ross River virus-induced disease in 15-day-old CD-1 mice. Fifteen-day-old CD-1 mice were infected with 103 PFU of RRV (Δ) by subcutaneous injection in the left rear footpad. Mock-infected mice (□) were injected with diluent alone. (A) Mice were scored for development of hind limb dysfunction and disease based on the following scale: 0, no disease signs; 1, ruffled fur; 2, very mild hind limb weakness; 3, mild hind limb weakness; 4, moderate hind limb weakness and dragging of hind limbs; 5, severe hind limb weakness/dragging; 6, complete loss of hind limb function; 7, moribund; and 8, death. (B) Mice were monitored for weight gain or loss at 24-h intervals. Each data point represents the arithmetic mean ± standard deviation (SD) for 7 (mock-infected) or 25 (RRV-infected) mice.
FIG. 2.
Ross River virus tissue titers in 15-day-old CD-1 mice. Fifteen-day-old CD-1 mice were infected with 103 PFU of RRV by subcutaneous injection in the left rear footpad. At 12, 24, 48, 72, 96, and 120 hpi, ankle (A), quadriceps muscle (B), serum (C), brain (D), and spleen (E) were harvested and homogenized, and the amount of infectious virus present was quantified by plaque assay on BHK-21 cells. Each data point represents the arithmetic mean ± SD for three mice.
FIG. 3.
Ross River virus replication within bone and joint-associated connective tissues and skeletal muscle tissue. Fifteen-day-old CD-1 mice were infected with 103 PFU of RRV by subcutaneous injection in the ventral thorax. Mock-infected mice were injected with diluent alone. Mice were sacrificed at 24 (A, B, C), 48 (D, E, F), and 72 (G, H, I) hours postinfection and perfused with 4% paraformaldehyde. Following decalcification, 5-μm-thick paraffin-embedded sections derived from the hind limbs were probed with 35S-labeled riboprobes complementary for RRV (A-I) or the EBER2 gene from Epstein-Barr virus (data not shown). M, muscle; B, bone. (A) RRV-specific in situ signal in tarsal bone periosteum. (B) RRV-specific in situ signal in synovial connective tissue of the knee joint. (C) Absence of RRV-specific in situ signal in hind limb skeletal muscle. (D) RRV-specific in situ signal in tarsal bone periosteum and associated skeletal muscle. (E) RRV-specific in situ signal in synovial tissue of a tarsal joint. (F) RRV-specific in situ signal in hind limb skeletal muscle tissue. (G) RRV-specific in situ signal in metatarsal bone periosteum and associated skeletal muscle. (H) RRV-specific in situ signal in hind limb tendon. (I) RRV-specific in situ signal in hind limb skeletal muscle.
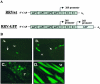
FIG. 4.
Ross River virus double-promoter virus targets cells within joint, bone, and skeletal muscle tissues. (A) Schematic diagram of Ross River virus that was engineered to express EGFP by inserting a second RRV 26S subgenomic promoter at the 3′ end of the viral genome followed by the EGFP coding sequence. (B) Ten- to 12-day-old CD-1 mice were infected with 103 PFU of RRV-EGFP by subcutaneous injection in the ventral thorax. At 48 and 72 hpi, tissues were harvested and sections were analyzed for EGFP expression. SC, synovial cavity; M, muscle; P, periosteum. The white arrows indicate the articular surface. (Subpanel A) Synovial cavity in the foot at 48 hpi. (Subpanel B) Synovial cavity in the ankle at 48 hpi. (Subpanel C) Periosteum in the hind limb at 48 hpi. (Subpanel D) Hind limb skeletal muscle at 72 hpi.
FIG. 5.
Ross River virus infection induces severe hind limb disease in 24-day-old C57BL/6J mice. Twenty-four-day-old C57BL/6J mice were inoculated with 103 PFU of RRV (Δ) by subcutaneous injection in the left rear footpad. Mock-infected mice (□) were injected with diluent alone. (A) Mice were scored for development of hind limb dysfunction and disease based on the following scale: 0, no disease signs; 1, ruffled fur; 2, very mild hind limb weakness; 3, mild hind limb weakness; 4, moderate hind limb weakness and dragging of hind limbs; 5, severe hind limb weakness/dragging; 6, complete loss of hind limb function; 7, moribund; and 8, death. (B) Mice were monitored for weight gain or loss at 24-h intervals. Each data point represents the arithmetic mean ± SD for three (mock-infected) or six (RRV-infected) animals. Data are representative of four independent experiments.
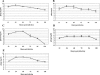
FIG. 6.
Ross River virus tissue titers in 24-day-old C57BL/6J mice. Twenty-four-day-old C57BL/6J mice were infected with 103 PFU of RRV by subcutaneous injection in the left rear footpad. At 12, 24, 48, 72, 96, and 120 hpi, the following tissues were harvested and homogenized and the amount of infectious virus present was quantified by plaque assay on BHK-21 cells. (A) □, ankle of injected leg; Δ, ankle of noninjected leg. (B) □, quadriceps muscle of injected leg; Δ, quadriceps muscle of noninjected leg. (C) Serum. (D) Spleen. (E) Brain. (F) □, lower spinal cord; Δ, upper spinal cord. Each data point represents the arithmetic mean ± SD for three mice.
FIG. 7.
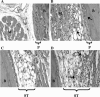
Ross River virus induces inflammation in hind limb bone and joint tissues of C57BL/6J mice. Twenty-four-day-old C57BL/6J mice were infected with 103 PFU of RRV by subcutaneous injection in the left rear footpad. Mock-infected mice were injected with diluent alone. M, muscle; B, bone; P, periosteum; ST, synovial tissue. At 5 days (A and B) and 7 days (C and D) postinfection, mice were perfused with 4% paraformaldehyde. Following decalcification, 5-μm-thick paraffin-embedded sections generated from ankle and foot tissues of mock-infected (A and C) and RRV-infected (B and D) mice were H & E stained. Images (magnification, ×200) are representative of at least six mice per group.
FIG. 8.
Ross River virus induces inflammation in hind limb skeletal muscle tissue of C57BL/6J mice. Twenty-four-day-old C57BL/6J mice were infected with 103 PFU of RRV by subcutaneous injection in the left rear footpad. Mock-infected mice were injected with diluent alone. At 3, 5, 7, 10, and 30 dpi, mice were perfused with 4% paraformaldehyde and 5-μm-thick paraffin-embedded sections generated from the quadriceps muscle were H & E stained. (A) Mock infection. (B) RRV infection at 3 dpi. (C) RRV infection at 5 dpi. (D) RRV infection at 7 dpi. (E) RRV infection at 10 dpi. (F) RRV infection at 30 dpi. Images (magnification, ×200) are representative of three to six mice per group.
FIG. 9.
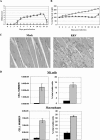
Characterization of Ross River virus-induced inflammatory infiltrates. Twenty-four-day-old C57BL/6J mice were infected with 103 PFU of RRV by subcutaneous injection in the left rear footpad. Mock-infected mice were injected with diluent alone. (A) Cell surface staining of cells isolated from the quadriceps muscle at 5 dpi. Dot plots shown for each stain are representative of three mice. Two independent experiments gave similar results. (B) Cell surface staining of cells isolated from the quadriceps muscle at 7 dpi. Dot plots shown for each stain are representative of three mice per group. Three independent experiments gave similar results. (C) Total numbers of natural killer cells (NK1.1+/CD3+), inflammatory macrophages (F4/80+/Gr-1+), CD4 T lymphocytes (CD3+/CD4+), and CD8 T lymphocytes (CD3+/CD8+) from the quadriceps muscle of mock- and RRV-infected animals at 5 and 7 days postinfection. Data presented are the means ± standard errors of the means for three to four mice per group and are representative of at least two independent experiments.
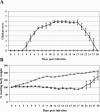
FIG. 10.
Ross River virus-induced inflammation and disease in RAG-1−/−. Twenty-four-day-old C57BL/6J RAG-1−/− mice were infected with 103 PFU of RRV (Δ) by subcutaneous injection in the left rear footpad. Mock-infected mice (□) were injected with diluent alone. (A) Mice were scored for development of hind limb dysfunction and disease based on the following scale: 0, no disease signs; 1, ruffled fur; 2, very mild hind limb weakness; 3, mild hind limb weakness; 4, moderate hind limb weakness and dragging of hind limbs; 5, severe hind limb weakness/dragging; 6, complete loss of hind limb function; 7, moribund; and 8, death. Each data point represents the arithmetic mean ± SD for two (mock-infected) or six (RRV-infected) mice and are representative of two independent experiments. (B) Mice were monitored for weight gain or loss at 24-h intervals. Each data point represents the arithmetic mean ± SD for four (mock-infected) or six (RRV-infected) mice and are representative of two independent experiments. (C) At 10 days postinfection, mice were perfused with 4% paraformaldehyde and 5-μm-thick paraffin-embedded sections generated from the quadriceps muscle were H & E stained. Images (magnification, ×200) are representative of three mice per group. (D) Total numbers and percentages of natural killer cells (NK1.1+/CD3−) and inflammatory macrophages (F4/80+/Gr-1+) from the quadriceps muscles of mock-infected (black bars) or RRV-infected (gray bars) RAG-1−/− mice at 7 days postinfection. Data presented are the means ± standard errors of the means for three mice per group and are representative of two independent experiments.
Similar articles
- Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease.
Morrison TE, Fraser RJ, Smith PN, Mahalingam S, Heise MT. Morrison TE, et al. J Virol. 2007 May;81(10):5132-43. doi: 10.1128/JVI.02799-06. Epub 2007 Feb 21. J Virol. 2007. PMID: 17314163 Free PMC article. - Mannose binding lectin is required for alphavirus-induced arthritis/myositis.
Gunn BM, Morrison TE, Whitmore AC, Blevins LK, Hueston L, Fraser RJ, Herrero LJ, Ramirez R, Smith PN, Mahalingam S, Heise MT. Gunn BM, et al. PLoS Pathog. 2012;8(3):e1002586. doi: 10.1371/journal.ppat.1002586. Epub 2012 Mar 22. PLoS Pathog. 2012. PMID: 22457620 Free PMC article. - CD8+ T cells control Ross River virus infection in musculoskeletal tissues of infected mice.
Burrack KS, Montgomery SA, Homann D, Morrison TE. Burrack KS, et al. J Immunol. 2015 Jan 15;194(2):678-89. doi: 10.4049/jimmunol.1401833. Epub 2014 Dec 8. J Immunol. 2015. PMID: 25488988 Free PMC article. - The molecular and cellular aspects of arthritis due to alphavirus infections: lesson learned from Ross River virus.
Rulli NE, Melton J, Wilmes A, Ewart G, Mahalingam S. Rulli NE, et al. Ann N Y Acad Sci. 2007 Apr;1102:96-108. doi: 10.1196/annals.1408.007. Ann N Y Acad Sci. 2007. PMID: 17470914 Review. - Ross River virus: molecular and cellular aspects of disease pathogenesis.
Rulli NE, Suhrbier A, Hueston L, Heise MT, Tupanceska D, Zaid A, Wilmes A, Gilmore K, Lidbury BA, Mahalingam S. Rulli NE, et al. Pharmacol Ther. 2005 Sep;107(3):329-42. doi: 10.1016/j.pharmthera.2005.03.006. Pharmacol Ther. 2005. PMID: 15923040 Review.
Cited by
- Role of pentraxin 3 in shaping arthritogenic alphaviral disease: from enhanced viral replication to immunomodulation.
Foo SS, Chen W, Taylor A, Sheng KC, Yu X, Teng TS, Reading PC, Blanchard H, Garlanda C, Mantovani A, Ng LF, Herrero LJ, Mahalingam S. Foo SS, et al. PLoS Pathog. 2015 Feb 19;11(2):e1004649. doi: 10.1371/journal.ppat.1004649. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25695775 Free PMC article. - Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses.
Shabman RS, Morrison TE, Moore C, White L, Suthar MS, Hueston L, Rulli N, Lidbury B, Ting JP, Mahalingam S, Heise MT. Shabman RS, et al. J Virol. 2007 Jan;81(1):237-47. doi: 10.1128/JVI.01590-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079324 Free PMC article. - Chikungunya virus replication in skeletal muscle cells is required for disease development.
Lentscher AJ, McCarthy MK, May NA, Davenport BJ, Montgomery SA, Raghunathan K, McAllister N, Silva LA, Morrison TE, Dermody TS. Lentscher AJ, et al. J Clin Invest. 2020 Mar 2;130(3):1466-1478. doi: 10.1172/JCI129893. J Clin Invest. 2020. PMID: 31794434 Free PMC article. - An attenuated replication-competent chikungunya virus with a fluorescently tagged envelope.
Jin J, Sherman MB, Chafets D, Dinglasan N, Lu K, Lee TH, Carlson LA, Muench MO, Simmons G. Jin J, et al. PLoS Negl Trop Dis. 2018 Jul 31;12(7):e0006693. doi: 10.1371/journal.pntd.0006693. eCollection 2018 Jul. PLoS Negl Trop Dis. 2018. PMID: 30063703 Free PMC article. - Methotrexate treatment causes early onset of disease in a mouse model of Ross River virus-induced inflammatory disease through increased monocyte production.
Taylor A, Sheng KC, Herrero LJ, Chen W, Rulli NE, Mahalingam S. Taylor A, et al. PLoS One. 2013 Aug 12;8(8):e71146. doi: 10.1371/journal.pone.0071146. eCollection 2013. PLoS One. 2013. PMID: 23951095 Free PMC article.
References
- Charge, S. B., and M. A. Rudnicki. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84:209-238. - PubMed
- Dalgarno, L., C. M. Rice, and J. H. Strauss. 1983. Ross River virus 26 s RNA: complete nucleotide sequence and deduced sequence of the encoded structural proteins. Virology 129:170-187. - PubMed
- Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reise Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. - PubMed
- Doherty, R. L., R. H. Whitehead, B. M. Gorman, and A. K. O'Gower. 1963. The isolation of a third group A arbovirus in Australia, with preliminary observations on its relationship to epidemic polyarthritis. Aust. J. Sci. 26:183-184.
Publication types
MeSH terms
Substances
Grants and funding
- F32 AR052600/AR/NIAMS NIH HHS/United States
- R01 AR047190/AR/NIAMS NIH HHS/United States
- F32 AR052600-01/AR/NIAMS NIH HHS/United States
- R01 AR47190/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials