Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a) - PubMed (original) (raw)
. 2005 Dec 27;102(52):19057-62.
doi: 10.1073/pnas.0509736102.
Francesca Chamian, Maria Veronica Abello, Judilyn Fuentes-Duculan, Shao-Lee Lin, Rachel Nussbaum, Inna Novitskaya, Henrietta Carbonaro, Irma Cardinale, Toyoko Kikuchi, Patricia Gilleaudeau, Mary Sullivan-Whalen, Knut M Wittkowski, Kim Papp, Marvin Garovoy, Wolfgang Dummer, Ralph M Steinman, James G Krueger
Affiliations
- PMID: 16380428
- PMCID: PMC1323218
- DOI: 10.1073/pnas.0509736102
Increase in TNF-alpha and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a)
Michelle A Lowes et al. Proc Natl Acad Sci U S A. 2005.
Abstract
We find that CD11c(+) cells with many markers of dendritic cells (DCs) are a major cell type in the skin lesions of psoriasis. These CD11c(+) cells, which are evident in both epidermis and dermis, are the sites for the expression of two mediators of inflammation, inducible nitric oxide synthase (iNOS) and TNF-alpha in diseased skin. These cells express HLA-DR, CD40, and CD86, lack the Langerin and CD14 markers of Langerhans cells and monocytes, respectively, and to a significant extent express the DC maturation markers DC-LAMP and CD83. Treatment of psoriasis with efalizumab (anti-CD11a, Raptiva) strongly reduces infiltration by these DCs in patients responding to this agent. Disease activity after therapy was more related to DC infiltrates and iNOS mRNA levels than T cell infiltrates, and CD11c(+) cells responded more quickly to therapy than epidermal keratinocytes. Our results suggest that a type of DC, which resembles murine "Tip-DCs" that can accumulate during infection, has proinflammatory effects in psoriasis through nitric oxide and TNF-alpha production, and can be an important target for suppressive therapies.
Figures
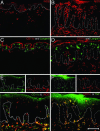
Fig. 1.
iNOS and TNF double-positive CD11c+ cells are greatly increased in psoriasis lesions. Single- and double-label immunofluorescence are shown. (Scale bar, 200 μm.) (Insets) Single color staining controls. (A) iNOS+ cells in the dermis of normal skin. (B) iNOS+ cells in the epidermis and dermis of psoriasis skin. (C) CD11c+ cells in the dermis of normal skin. (D) CD11c+ cells in the epidermis and dermis of psoriasis skin. No overlap with Langerin positive cells in the epidermis in C and D.(E and F) There is nearly complete overlap between CD11c and iNOS, and CD11c and TNF.
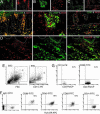
Fig. 2.
CD11c identifies a type of DC. (_A_-D) Double-label immunofluorescence. (Insets) Single-color staining controls. (A) CD11c+ cells are all HLA-DR+.(B and C) Some iNOS+ cells coproduce DC-LAMP and CD83. DC-LAMP and iNOS+ cells are mature dermal DCs. (D) TNF+ cells are predominantly HLA-DR+. (_E_-G) FACS of dermal emigrants from a psoriatic patient. (E) R1, cells gated based on large cell size (FSC) and high side scatter (SCC); R2, CD11c-positive cells. (F) HLA-DR versus IgG1, CD86, CD40, CD83 and CD14 expression (R1+R2). Percent double-positive cells is shown in the upper right quadrant. (G) A lymphocyte gate (R3) indicates CD3-positive cells that have low CD11c and CD83 staining.
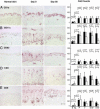
Fig. 3.
CD11c+ DCs are significantly decreased after efalizumab (anti-CD11a) treatment. Histomicrographs and cell counts of skin biopsies from a normal control and a treatment group patient (responder, K16 negative), on days 0 and 56. (Scale bar, 200 μm.) Mean number of total cells per low power field (10×) for CD1a (A), CD11c (B), CD83 (C), CD3 (D), and CD8 (E) (±SD) in normal skin (N, black), nonlesional psoriasis skin (NL, gray), and lesional skin of patients in placebo (P), treatment (T; all patients), K16+ (non- and partial responders), and K16- (high responders) groups on days 0 (filled squares) and 56 (open squares). P values are shown for the indicated comparison.
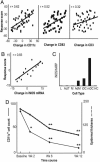
Fig. 4.
Changes in number of CD11c and CD83 positive cells, as well as iNOS mRNA expression, correlate highly with disease improvement. (A) Correlation between response score and changes in DCs (CD11c+ and CD83+) and T cells (CD3+)(u score) between day 0 and 56 for patients in the efalizumab-treated group. Correlation coefficients (r) are shown. (B) Correlation between response score and change in iNOS mRNA in lesional tissue between day 0 and 56 (r = 0.83). (C) In vitro monocyte-derived DCs produce iNOS mRNA. INOS/HARP mRNA was measured by RT-PCR in lymphocytes (L), T cells activated by CD3/CD28 (ActT), monocytes (M), adherent monocytes (AdM), immature DCs (iDC), mature dendritic cells (mDC), and HaCaT cells (HC). (D) Dermal CD11c+ cell counts/low power field (straight black line) and epidermal thickness (dotted black line) during therapy. During the first 2 weeks of treatment, there is 50% reduction in dermal CD11c+ cells (*, P = 0.006) and a 14% reduction in epidermal thickness (not significant). **, CD11c+ cell counts and epidermal thickness at weeks 6 and 12 compared to baseline, P < 0.0001.
Similar articles
- Infliximab induces downregulation of the IL-12/IL-23 axis in 6-sulfo-LacNac (slan)+ dendritic cells and macrophages.
Brunner PM, Koszik F, Reininger B, Kalb ML, Bauer W, Stingl G. Brunner PM, et al. J Allergy Clin Immunol. 2013 Nov;132(5):1184-1193.e8. doi: 10.1016/j.jaci.2013.05.036. Epub 2013 Jul 26. J Allergy Clin Immunol. 2013. PMID: 23890755 - Generation and functional analysis of human TNF-α/iNOS-producing dendritic cells (Tip-DC).
Wilsmann-Theis D, Koch S, Mindnich C, Bonness S, Schnautz S, von Bubnoff D, Bieber T. Wilsmann-Theis D, et al. Allergy. 2013 Jul;68(7):890-8. doi: 10.1111/all.12172. Epub 2013 Jun 6. Allergy. 2013. PMID: 23742057 - The Origin of Skin Dendritic Cell Network and Its Role in Psoriasis.
Kim TG, Kim SH, Lee MG. Kim TG, et al. Int J Mol Sci. 2017 Dec 23;19(1):42. doi: 10.3390/ijms19010042. Int J Mol Sci. 2017. PMID: 29295520 Free PMC article. Review. - The pathophysiological role of dendritic cell subsets in psoriasis.
Kim TG, Kim DS, Kim HP, Lee MG. Kim TG, et al. BMB Rep. 2014 Feb;47(2):60-8. doi: 10.5483/bmbrep.2014.47.2.014. BMB Rep. 2014. PMID: 24411465 Free PMC article. Review.
Cited by
- Immunology of psoriasis.
Lowes MA, Suárez-Fariñas M, Krueger JG. Lowes MA, et al. Annu Rev Immunol. 2014;32:227-55. doi: 10.1146/annurev-immunol-032713-120225. Annu Rev Immunol. 2014. PMID: 24655295 Free PMC article. Review. - Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment.
Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen JD, Parmentier M, De Pità O, Girolomoni G, Sozzani S. Albanesi C, et al. J Exp Med. 2009 Jan 16;206(1):249-58. doi: 10.1084/jem.20080129. Epub 2008 Dec 29. J Exp Med. 2009. PMID: 19114666 Free PMC article. - Phenotype, Function, and Mobilization of 6-Sulfo LacNAc-Expressing Monocytes in Atopic Dermatitis.
Baran W, Oehrl S, Ahmad F, Döbel T, Alt C, Buske-Kirschbaum A, Schmitz M, Schäkel K. Baran W, et al. Front Immunol. 2018 Jun 21;9:1352. doi: 10.3389/fimmu.2018.01352. eCollection 2018. Front Immunol. 2018. PMID: 29977237 Free PMC article. - Genomic Profiling of the Overlap Phenotype between Psoriasis and Atopic Dermatitis.
Kim JE, Lee J, Huh YJ, Kim K, Chaparala V, Krueger JG, Kim J. Kim JE, et al. J Invest Dermatol. 2024 Jan;144(1):43-52.e6. doi: 10.1016/j.jid.2023.06.194. Epub 2023 Jul 5. J Invest Dermatol. 2024. PMID: 37419444 Free PMC article. - Nitric oxide controls an inflammatory-like Ly6C(hi)PDCA1+ DC subset that regulates Th1 immune responses.
Giordano D, Li C, Suthar MS, Draves KE, Ma DY, Gale M Jr, Clark EA. Giordano D, et al. J Leukoc Biol. 2011 Mar;89(3):443-55. doi: 10.1189/jlb.0610329. Epub 2010 Dec 22. J Leukoc Biol. 2011. PMID: 21178115 Free PMC article.
References
- Krueger, J. G. (2002) J. Am. Acad. Dermatol. 46, 1-23. - PubMed
- Lew, W., Bowcock, A. M. & Krueger, J. G. (2004) Trends Immunol. 25, 295-305. - PubMed
- Papp, K., Bissonnette, R., Krueger, J. G., Carey, W., Gratton, D., Gulliver, W. P., Lui, H., Lynde, C. W., Magee, A., Minier, D., et al. (2001) J. Am. Acad. Dermatol. 45, 665-674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI-49572/AI/NIAID NIH HHS/United States
- R01 AI049572/AI/NIAID NIH HHS/United States
- M01 RR000102/RR/NCRR NIH HHS/United States
- R01 AI049832/AI/NIAID NIH HHS/United States
- AI-49832/AI/NIAID NIH HHS/United States
- M01-RR00102/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials