CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum - PubMed (original) (raw)
CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum
Amir A Levine et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Remodeling chromatin is essential for cAMP-regulated gene expression, necessary not only for development but also for memory storage and other enduring mental states. Histone acetylation and deacetylation mediate long-lasting forms of synaptic plasticity in Aplysia as well as cognition in mice. Here, we show that histone acetylation by the cAMP-response element binding protein (CREB)-binding protein (CBP) mediates sensitivity to cocaine by regulating expression of the fosB gene and its splice variant, DeltafosB, a transcription factor previously implicated in addiction. Using the chromatin immunoprecipitation assay with antibodies against histone H4 or CBP, we find that CBP is recruited to the fosB promoter to acetylate histone H4 in response to acute exposure to cocaine. We show that mutant mice that lack one allele of the CBP gene and have normal levels of fosB expression are less sensitive to chronic (10-day) administration of cocaine than are wild-type mice. This decreased sensitivity is correlated with decreased histone acetylation and results in decreased fosB expression and diminished accumulation of DeltafosB. Thus, CBP, which forms part of the promoter complex with CREB, mediates sensitivity to cocaine by acetylating histones.
Figures
Fig. 1.
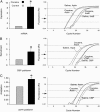
Cocaine induces fosB expression through CBP recruitment and histone acetylation at the fosB promoter. (A) Real-time RT-PCR shows that fosB is induced in C57BL/6J mouse striata 2 h after injecting 30 mg/kg cocaine i.p. (n = 3 in each group; actin set at 1; cocaine, 7.88 ± 0.15; P < 0.001). (B) ChIP assays show increased CBP recruitment to the fosB promoter 20 min after injecting 30 mg/kg cocaine i.p. [n = 7 in each group; control set at 1; cocaine, 2.45 ± 0.65; P < 0.05, input (nonimmunoprecipitated DNA) was used as control]. (C) The acetylation of histone H4 at the fosB promoter was increased 20 min after injecting 30 mg/kg cocaine i.p. (n = 6 mice pairs; control set at 1; cocaine, 1.41 ± 0.14; P < 0.05). Normalized reporter (Rn) represents the fluorescence detected. Data are expressed as mean ± SEM, P values were measured by Student's t test. Representative data from real-time PCR experiments are shown to the right of each bar chart. The quantifying of fosB cDNA (A), and CBP and acetylated histone H4 antibody pulldown (B and C) was done by normalizing Ct values (Ct value is the number of cycles at which the fluorescence crosses a threshold indicated by the arrow at the abscissa) of cDNA and immunoprecipitated DNA (saline control vs. cocaine) to Ct values of actin and input DNA. Acetylated histone H4 at the actin promoter and levels of actin cDNA were unaffected by cocaine.
Fig. 2.
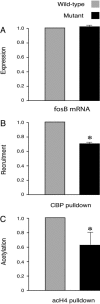
Basal recruitment of CBP and histone H4 acetylation at the fosB promoter in CBP haploinsufficient and in wild-type mice. (A) The basal expression of fosB in the mutant and wild-type were equivalent (n = 3 pairs; wild type set at 1; mutant, 1.061 ± 0.041; P > 0.05). Note that the values are set relative to those of the wild-type. The fosB expression was 4- to 8-fold less than that observed after treatment with cocaine (also see Fig. 1_A_). (B) CBP recruitment was less in the mutant (n = 5 in each group, wild type set at 1; mutant, 0.68 ± 0.06; P < 0.01; input DNA was used as control). (C) Basal histone H4 acetylation at the fosB promoter in the mutant also was less than that in the wild type (n = 5 in each group; wild type set at1; mutant, 0.63 ± 0.17; P < 0.05; input DNA was used as control).
Fig. 3.
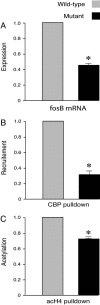
Cocaine-induced CBP recruitment and histone H4 acetylation are decreased at the fosB promoter, resulting in decreased fosB expression in CBP haploinsufficient mice. (A) Cocaine was less effective in inducing fosB in the mutant mice. Values were from the mice 2 h after injection of 30 mg/kg cocaine i.p. (n = 3 mice pairs, control, 1; cocaine, 0.448 ± 0.033; P < 0.01). (B and C) ChIP assays show that, 20 min after 30 mg/kg cocaine injection, there is a decrease in CBP recruitment to the fosB promoter in mutant mouse striatum compared to wild type (n = 3 in each group, wild type set at 1, mutant 0.31; P < 0.05, input DNA as control) (B) and a decrease in acetylated histone H4 at the fosB promoter (n = 3 in each group; wild type set at 1; mutant 0.67; P = 0.014, input DNA as control) (C). Data are expressed as mean ± SEM.
Fig. 4.
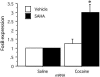
Expression of fosB in the striatum of mice treated with a histone deacetylase inhibitor is enhanced after cocaine treatment. Real-time PCR shows a 3-fold increase in fosB expression after cocaine injection in mice that were pretreated with SAHA. Treatment with SAHA without cocaine administration did not change fosB expression. C57BL/6J were injected with SAHA (25 mg/kg) or vehicle (DMSO) 2 h before receiving cocaine (20 mg/kg). Mice were killed 2 h after the injection of cocaine. n = 18; control set at 1; DMSO and cocaine, 1.25 ± 0.001; SAHA plus cocaine, 3 ± 0.002; *, P < 0.05.
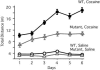
Fig. 5.
CBP haploinsufficient mice show decreased sensitivity to cocaine. Sensitivity assessed by locomotor activity: total (±SEM) distance traveled (in meters) of wild-type and CBP haploinsufficient mice during six consecutive days of cocaine injection [cocaine: 30 mg/kg: +/+, n = 7; +/-, n = 7; vehicle (saline): +/+, n = 7; +/-, n = 6]. There was no difference in activity between mutant and wild-type groups that were injected with saline. Activity was greater in wild-type than in cocaine-injected CBP haploinsufficient mice (P < 0.05 for all days), whereas there was no difference between the wild-type and mutant controls.
Similar articles
- CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors.
Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. Malvaez M, et al. J Neurosci. 2011 Nov 23;31(47):16941-8. doi: 10.1523/JNEUROSCI.2747-11.2011. J Neurosci. 2011. PMID: 22114264 Free PMC article. - Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum.
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Kumar A, et al. Neuron. 2005 Oct 20;48(2):303-14. doi: 10.1016/j.neuron.2005.09.023. Neuron. 2005. PMID: 16242410 - Epigenetic landscape of amphetamine and methamphetamine addiction in rodents.
Godino A, Jayanthi S, Cadet JL. Godino A, et al. Epigenetics. 2015;10(7):574-80. doi: 10.1080/15592294.2015.1055441. Epigenetics. 2015. PMID: 26023847 Free PMC article. Review. - Molecular neurobiology of addiction.
Nestler EJ. Nestler EJ. Am J Addict. 2001 Summer;10(3):201-17. doi: 10.1080/105504901750532094. Am J Addict. 2001. PMID: 11579619 Review.
Cited by
- Psychostimulants and opioids differentially influence the epigenetic modification of histone acetyltransferase and histone deacetylase in astrocytes.
Doke M, Pendyala G, Samikkannu T. Doke M, et al. PLoS One. 2021 Jun 11;16(6):e0252895. doi: 10.1371/journal.pone.0252895. eCollection 2021. PLoS One. 2021. PMID: 34115777 Free PMC article. - Unique functional roles for class I and class II histone deacetylases in central nervous system development and function.
Morris MJ, Monteggia LM. Morris MJ, et al. Int J Dev Neurosci. 2013 Oct;31(6):370-81. doi: 10.1016/j.ijdevneu.2013.02.005. Epub 2013 Mar 4. Int J Dev Neurosci. 2013. PMID: 23466417 Free PMC article. Review. - Differential effects of cocaine on histone posttranslational modifications in identified populations of striatal neurons.
Jordi E, Heiman M, Marion-Poll L, Guermonprez P, Cheng SK, Nairn AC, Greengard P, Girault JA. Jordi E, et al. Proc Natl Acad Sci U S A. 2013 Jun 4;110(23):9511-6. doi: 10.1073/pnas.1307116110. Epub 2013 May 20. Proc Natl Acad Sci U S A. 2013. PMID: 23690581 Free PMC article. - Combinatorial chromatin modifications and memory storage: a code for memory?
Wood MA, Hawk JD, Abel T. Wood MA, et al. Learn Mem. 2006 May-Jun;13(3):241-4. doi: 10.1101/lm.278206. Learn Mem. 2006. PMID: 16741277 Free PMC article. Review. No abstract available. - Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference.
Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Malvaez M, et al. Biol Psychiatry. 2010 Jan 1;67(1):36-43. doi: 10.1016/j.biopsych.2009.07.032. Biol Psychiatry. 2010. PMID: 19765687 Free PMC article.
References
- Kandel, E. R. & Schwartz, J. H. (1982) Science 218, 433-443. - PubMed
- Bartsch, D., Casadio, A., Karl, K. A., Serodio, P. & Kandel, E. R. (1998) Cell 95, 211-223. - PubMed
- Guan, Z., Giustetto, M., Lomvardas, S., Kim, J. H., Miniaci, M. C., Schwartz, J. H., Thanos, D. & Kandel, E. R. (2002) Cell 111, 483-493. - PubMed
- Alberini, C. M., Ghirardi, M., Metz, R. & Kandel, E. R. (1994) Cell 76, 1099-1114. - PubMed
- Hegde, A. N., Inokuchi, K., Pei, W., Casadio, A., Ghirardi, M., Chain, D. G., Martin, K. C., Kandel, E. R. & Schwartz, J. H. (1997) Cell 89, 115-126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS29255/NS/NINDS NIH HHS/United States
- R01 MH060387/MH/NIMH NIH HHS/United States
- R01 MH048850/MH/NIMH NIH HHS/United States
- R01 NS029255/NS/NINDS NIH HHS/United States
- MH60387/MH/NIMH NIH HHS/United States
- MH048850/MH/NIMH NIH HHS/United States
- T32 MH015174/MH/NIMH NIH HHS/United States
- MH15174/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous