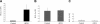Role of endothelial heparanase in delayed-type hypersensitivity - PubMed (original) (raw)
Role of endothelial heparanase in delayed-type hypersensitivity
Evgeny Edovitsky et al. Blood. 2006.
Abstract
Heparanase is an endoglycosidase that cleaves heparan sulfate (HS), the main polysaccharide of the basement membrane (BM). HS is responsible for BM integrity and barrier function. Hence, enzymatic degradation of HS in the vascular subendothelial BM is a prerequisite for extravasation of immune cells and plasma components during inflammation. Here, we demonstrate a highly coordinated local heparanase induction upon elicitation of delayed-type hypersensitivity (DTH) reaction in the mouse ear. By monitoring in vivo activation of luciferase gene driven by the heparanase promoter, we demonstrate activation of heparanase transcription at an early stage of DTH. We report that heparanase is produced locally by the endothelium at the site of DTH-associated inflammation. Key DTH mediators, tumor necrosis factor-alpha and interferon-gamma, were found to induce heparanase in cultured endothelial cells. Endothelium emerges as an essential cellular source of heparanase enzymatic activity that, in turn, allows for remodeling of the vascular BM, increased vessel permeability, and extravasation of leukocytes and plasma proteins. In vivo administration of antiheparanase siRNA or an inhibitor of heparanase enzymatic activity effectively halted DTH inflammatory response. Collectively, our results highlight the decisive role of endothelial heparanase in DTH inflammation and its potential as a promising target for anti-inflammatory drug development.
Figures
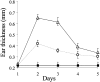
Figure 1.
Increased DTH reactivity in heparanase overexpressing transgenic mice. DTH reactions were elicited in the left ear skin of _hpa_-tg mice and their wild-type counterparts using oxazolone. Right ears of the same animals were treated with vehicle alone. Swelling of the challenged ears is expressed in mm as the increase over the baseline thickness measured in ears treated with vehicle alone. Challenged ears in _hpa_-tg mice (▵) showed a 3.5-fold increase in swelling over the baseline (▪), as compared to only 2-fold increase in wild-type mice (○), 24 hours after challenge with oxazolone. The differences between the 2 groups remained statistically significant for 3 days (n = 5 per experimental condition and time point). Data are expressed as mean ± SD. The experiment was repeated twice with similar results.
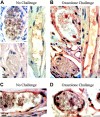
Figure 2.
Heparanase expression in vivo upon DTH induction. (A, B) Endogenous heparanase: 5 days after sensitization, left ear (B) of female BALB/c mice (n = 4) was treated with oxazolone and the right ear (A) with vehicle alone. Ear tissues were harvested 24 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining). Vascular structures were recognized as luminal or slit-like structures that occasionally contained blood cells and were delineated by flattened endothelial cells. This experiment was repeated 3 times, and a similar immunostaining pattern was obtained with 2 different antiheparanase antibodies. Representative microphotographs are shown. (A) Nonchallenged ear: capillary endothelial cells in the ear skin dermis are negative for heparanase staining (magnification × 1000). (B) Oxazolone-challenged ear: heparanase-expressing capillary endothelial cells are easily detected (× 1000). Control sections stained using secondary antibody alone showed no staining. (C, D) When DTH reaction was elicited in _hpa_-tg mice, positive staining was detected in capillary endothelium both prior to (C) and after (D) the challenge. Images were captured with a Zeiss Axioskop 50 microscope (Zeiss, Oberkochen, Germany) equipped with 100 ×/1.30 oil objective or 20 ×/0.50 objective lenses. Images were captured with a Kodak DC290 digital camera (Kodak, Rochester, NY).
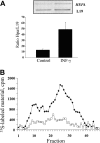
Figure 3.
Effects of IFN-γ on heparanase expression in endothelial cells. (A) Semiquantitative RT-PCR. EA.hy926 cells were incubated (16 hours) in triplicate in the absence or presence of 80 ng/mL IFN-γ. RNA was then isolated from the cells, and comparative semiquantitative PCR was performed as described in “Materials and methods.” Aliquots (10 μL) of the PCR products were separated by 1.5% agarose gel electrophoresis and visualized (top). The intensity of each band was quantitated using Scion Image software, and the results are expressed as band intensity relative to that of the housekeeping gene L19. The bars represent the mean ± SD (error bars) of 3 independent experiments (bottom). (B) Heparanase activity. EA.hy926 cells were incubated (16 hours) in the absence (□), or presence (♦) of 80 ng/mL IFN-γ. Cell lysates were normalized for equal protein and incubated (3 hours, pH 6.0, 37°C) with sulfate-labeled ECM. Labeled degradation fragments released into the incubation medium were analyzed by gel filtration on sepharose 6B.
Figure 4.
Heparanase promoter is activated upon DTH elicitation. The ears of oxazolone-sensitized BALB/c mice (n = 3) were electroporated with Hpse-LUC (A, experimental group), CMV-LUC (B, control group), or Mut-Hpse-LUC (C, control group) reporter constructs. Left ears in both the experimental and control groups were challenged 24 hours later, while right ears remained untreated. Forty-eight hours after challenge, when a pronounced DTH reaction was noted in the left, but not right, ears of all mice (as judged by ear swelling and edema formation), the ears were resected, snap frozen, and lysed. Lysates were normalized for total protein content. Luciferase activity was determined as described in “Materials and methods” and expressed in relative units of light (RUL). Two independent experiments were performed, 3 mice per treatment.
Figure 5.
Effect of antiheparanase siRNA on DTH reactivity in vivo. Ears of oxazolone-sensitized BALB/c mice were electroporated with anti–mouse heparanase siRNA expression vector pSi2 (▪); empty vector pSUPER (▴); or received no plasmid or electroporation (♦), followed by challenge with the hapten 24 hours later. Hapten also was applied on the ears of 5 additional mice, which have not been previously sensitized or electroporated (▪). Three independent experiments were performed, and 5 mice per treatment were used. (A) Ear thickness was measured for 5 consecutive days after challenge. Inset. Effect of treatment with an inhibitor (ST1514) of heparanase enzymatic activity on DTH reactivity. ST1514 or vehicle alone was administered intraperitoneally prior to challenge and every hour thereafter (50 μg/injection) during the following 8 hours of the experiment. Filled bars: ear thickness in ST1514-treated mice; empty bars: ear thickness in vehicle-treated mice. (B) The ears in which DTH was induced following electroporation with pSi2 (left) or pSUPER (right) vectors were harvested 48 hours after challenge and processed for immunohistochemical analysis of heparanase expression (reddish staining; sebaceous glands are positively stained in all preparates, due to nonspecific absorption, as previously reported. Top: magnification × 200; bottom: × 1000. Positively stained capillary endothelium is noted in the dermis of pSUPER but not pSi2-electroporated ear skin. (C) To demonstrate that electroporation ensures the actual delivery of plasmid DNA and its uniform expression in the ear tissue, the ears of male BALB/c mice were electroporated with a CMV-LUC construct, encoding for luciferase gene under the constitutive CMV promoter. Expression of luciferase in the mouse ears in vivo was monitored as described in “Materials and methods.”
Figure 6.
Effect of local heparanase silencing on vascular leakage. Ears of 5 oxazolone-sensitized BALB/c mice were electroporated with either antiheparanase siRNA pSi2 (filled arrows) or empty pSUPER (empty arrows) vectors, 24 hours prior to induction of DTH reaction by application of oxazolone onto the ears of both sides. Evans blue dye was injected intravenously 16 hours later. Unlike the massive Evans blue extravasation observed in pSUPER-electroporated ears, pSi2 electroporation halted vascular leakage, as visualized by the near absence of extravasated Evans blue dye.
Similar articles
- Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression.
Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ. Chen G, et al. Biochemistry. 2004 May 4;43(17):4971-7. doi: 10.1021/bi0356552. Biochemistry. 2004. PMID: 15109255 - Heparanase of murine effector lymphocytes and neutrophils is not required for their diapedesis into sites of inflammation.
Stoler-Barak L, Petrovich E, Aychek T, Gurevich I, Tal O, Hatzav M, Ilan N, Feigelson SW, Shakhar G, Vlodavsky I, Alon R. Stoler-Barak L, et al. FASEB J. 2015 May;29(5):2010-21. doi: 10.1096/fj.14-265447. Epub 2015 Jan 29. FASEB J. 2015. PMID: 25634957 - Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis.
Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I. Edovitsky E, et al. J Natl Cancer Inst. 2004 Aug 18;96(16):1219-30. doi: 10.1093/jnci/djh230. J Natl Cancer Inst. 2004. PMID: 15316057 - Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis.
Ilan N, Elkin M, Vlodavsky I. Ilan N, et al. Int J Biochem Cell Biol. 2006;38(12):2018-39. doi: 10.1016/j.biocel.2006.06.004. Epub 2006 Jul 6. Int J Biochem Cell Biol. 2006. PMID: 16901744 Review. - Heparanase: Historical Aspects and Future Perspectives.
Khanna M, Parish CR. Khanna M, et al. Adv Exp Med Biol. 2020;1221:71-96. doi: 10.1007/978-3-030-34521-1_3. Adv Exp Med Biol. 2020. PMID: 32274707 Review.
Cited by
- Vascular endothelial growth factor c/vascular endothelial growth factor receptor 3 signaling regulates chemokine gradients and lymphocyte migration from tissues to lymphatics.
Iwami D, Brinkman CC, Bromberg JS. Iwami D, et al. Transplantation. 2015 Apr;99(4):668-77. doi: 10.1097/TP.0000000000000561. Transplantation. 2015. PMID: 25606800 Free PMC article. - Heparanase: a target for drug discovery in cancer and inflammation.
McKenzie EA. McKenzie EA. Br J Pharmacol. 2007 May;151(1):1-14. doi: 10.1038/sj.bjp.0707182. Epub 2007 Mar 5. Br J Pharmacol. 2007. PMID: 17339837 Free PMC article. Review. - Acute T-Cell-Driven Inflammation Requires the Endoglycosidase Heparanase-1 from Multiple Cell Types.
Wu Z, Sweet RA, Hoyne GF, Simeonovic CJ, Parish CR. Wu Z, et al. Int J Mol Sci. 2022 Apr 21;23(9):4625. doi: 10.3390/ijms23094625. Int J Mol Sci. 2022. PMID: 35563015 Free PMC article. - Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity.
Lanzi C, Cassinelli G. Lanzi C, et al. Molecules. 2018 Nov 8;23(11):2915. doi: 10.3390/molecules23112915. Molecules. 2018. PMID: 30413079 Free PMC article. Review. - Non-Anticoagulant Heparins as Heparanase Inhibitors.
Cassinelli G, Torri G, Naggi A. Cassinelli G, et al. Adv Exp Med Biol. 2020;1221:493-522. doi: 10.1007/978-3-030-34521-1_20. Adv Exp Med Biol. 2020. PMID: 32274724 Free PMC article. Review.
References
- Voisin GA, Toullet F. Studies on hypersensitivity, I: demonstration and description of an increase of vascular permeability in the tuberculin hypersensitivity reactions. Ann Inst Pasteur. 1963;104: 169-196. - PubMed
- Abbas AK. Cell-mediated (type IV) hypersensitivity. In: Kumar V, Abbas AK, Fausto N, eds. Pathologic Basis of Disease. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 216-217.
- Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5: 7. - PubMed
- Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67: 149-159. - PubMed
- Noonan DM, Fulle A, Valente P, et al. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266: 22939-22947. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases