Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons - PubMed (original) (raw)
Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons
Natalia Omelchenko et al. J Comp Neurol. 2006.
Abstract
Cholinergic afferents to the ventral tegmental area (VTA) contribute substantially to the regulation of motivated behaviors and the rewarding properties of nicotine. These actions are believed to involve connections with dopamine (DA) neurons projecting to the nucleus accumbens (NAc). However, this direct synaptic link has never been investigated, nor is it known whether cholinergic inputs innervate other populations of DA and gamma-aminobutyric acid (GABA) neurons, including those projecting to the prefrontal cortex (PFC). We addressed these questions by using electron microscopic analysis of retrograde tract-tracing and immunocytochemistry for the vesicular acetylcholine transporter (VAChT) and for tyrosine hydroxylase (TH) and GABA. In tissue labeled for TH, VAChT(+) terminals frequently synapsed onto DA mesoaccumbens neurons but only seldom contacted DA mesoprefrontal cells. In tissue labeled for GABA, one-third of VAChT(+) terminals innervated GABA-labeled dendrites, including both mesoaccumbens and mesoprefrontal populations. VAChT(+) synapses onto DA and mesoaccumbens neurons were more commonly of the asymmetric (presumed excitatory) morphological type, whereas VAChT(+) synapses onto GABA cells were more frequently symmetric (presumed inhibitory or modulatory). These findings suggest that cholinergic inputs to the VTA mediate complex synaptic actions, with a major portion of this effect likely to involve an excitatory influence on DA mesoaccumbens neurons. As such, the results suggest that natural and drug rewards operating through cholinergic afferents to the VTA have a direct synaptic link to the mesoaccumbens DA neurons that modulate approach behaviors.
J. Comp. Neurol. 494:863-875, 2006. (c) 2005 Wiley-Liss, Inc.
Figures
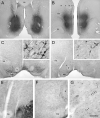
Figure 1
Light microscopic images of rat coronal brain sections. Panels A and B show injections of FG into the NAc or PFC. The corresponding retrograde transport of FG to the VTA is shown in panels C and D. Inserts illustrate the transport of FG into soma and dendrites (arrows indicate the same cells). Panels E-G illustrate peroxidase labeling for VAChT in the VTA and adjacent interpeduncular nucleus (IP). Although VAChT+ fibers are notably denser in the IP than in the VTA, VAChT axons in the latter structure viewed at higher magnification are markedly beaded and appear to form chains of varicosities (small arrows in G). Abbreviations: ac, anterior commissure; cc, corpus callosum; fm, forceps minor; fr, fasciculus retroflexus; LV, lateral ventricle; ml, medial lemniscus; mp, mammillary peduncle; rs, rhinal sulcus. Scale bar represents 1 mm in A-B, 500 μm in C-D, 62.5 in inserts, 125 μm in E-F, and 31.25 μm in G.
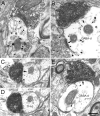
Figure 2
Electron micrographs of the rat VTA demonstrating immunoperoxidase labeled VAChT-positive axon terminals (VT) forming synapses with asymmetric (white arrow) or symmetric (black arrows) morphology onto unlabeled dendrites (ud). One VT terminal also shows immunogold-silver labeling for GABA (GABA/VT in C), and a GABA-labeled dendrite appears in the adjacent neuropil (GABA-d in B). Scale bar, 0.33 μm.
Figure 3
Electron micrographs showing asymmetric (white arrows) or symmetric (black arrows) synapses formed by VAChT-positive terminals (VT) onto dendrites singly labeled by immunogold-silver for TH (TH-d) or GABA (GABA-d). The GABA-d in B receives additional synaptic input from a GABA-labeled terminal (GABA-t). The TH-d in C is shown in a serial section in D to verify the TH labeling. Scale bar, 0.33 μm.
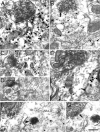
Figure 4
Electron micrographs of the VTA from rats receiving FG injections into the NAc. VAChT-immunoreactive terminals (VT) form asymmetric (white arrows) or symmetric (black arrows) synapses onto soma (s) or dendrites (d) dually labeled by immunoperoxidase for FG and immunogold-silver for TH (FG+TH) or GABA (FG+GABA). In C, the VT synapses onto a FG+TH soma, as evidenced by the presence of a nucleus (n). The FG+GABA-d in F contains a low level of GABA labeling and FG concentrated in a lysosome (arrowhead). The serial section in G shows the synapse formed by the VT. Scale bar, 0.33 μm for A-C and E-G; 0.5 μm for D.
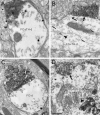
Figure 5
Electron micrographs of the VTA from rats receiving FG injections into the PFC. VAChT-labeled axon terminals (VT) form asymmetric (white arrows) or symmetric synapses (black arrows) onto dendrites dually-labeled for FG and TH (FG+TH-d) or FG and GABA (FG+GABA-d) or singly-labeled for FG (FG-d). In B, sparse immunogold-silver labeling for TH is indicated by small arrows. Arrowheads indicate diffuse FG in the cytoplasm (B) or concentrated in a lysosome (D). Scale bar, 0.33 μm for A, B, D; 0.40 μm for C.
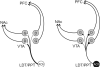
Figure 6
Schematic drawing comparing the asymmetric (presumed excitatory) synapses in white and symmetric (presumed modulatory/inhibitory) synapses in black formed by cholinergic axons presumably coming from the mesopontine tegmentum onto identified cell populations of DA (D) and GABA (G) VTA neurons. The thickness of cholinergic axons is weighted to reflect the approximate frequency of the connections observed in the present study.
Similar articles
- Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons.
Carr DB, Sesack SR. Carr DB, et al. J Neurosci. 2000 May 15;20(10):3864-73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. J Neurosci. 2000. PMID: 10804226 Free PMC article. - Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area.
Omelchenko N, Sesack SR. Omelchenko N, et al. J Comp Neurol. 2005 Mar 7;483(2):217-35. doi: 10.1002/cne.20417. J Comp Neurol. 2005. PMID: 15678476 - Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources.
Omelchenko N, Sesack SR. Omelchenko N, et al. Neuroscience. 2007 May 25;146(3):1259-74. doi: 10.1016/j.neuroscience.2007.02.016. Epub 2007 Mar 28. Neuroscience. 2007. PMID: 17391856 Free PMC article. - Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter.
Garzón M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Garzón M, et al. J Comp Neurol. 1999 Jul 26;410(2):197-210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. J Comp Neurol. 1999. PMID: 10414527 Review. - Cholinergic modulation of mesolimbic dopamine function and reward.
Mark GP, Shabani S, Dobbs LK, Hansen ST. Mark GP, et al. Physiol Behav. 2011 Jul 25;104(1):76-81. doi: 10.1016/j.physbeh.2011.04.052. Epub 2011 May 1. Physiol Behav. 2011. PMID: 21549724 Free PMC article. Review.
Cited by
- Knockouts reveal overlapping functions of M(2) and M(4) muscarinic receptors and evidence for a local glutamatergic circuit within the laterodorsal tegmental nucleus.
Kohlmeier KA, Ishibashi M, Wess J, Bickford ME, Leonard CS. Kohlmeier KA, et al. J Neurophysiol. 2012 Nov;108(10):2751-66. doi: 10.1152/jn.01120.2011. Epub 2012 Sep 5. J Neurophysiol. 2012. PMID: 22956788 Free PMC article. - Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior.
Picciotto MR, Higley MJ, Mineur YS. Picciotto MR, et al. Neuron. 2012 Oct 4;76(1):116-29. doi: 10.1016/j.neuron.2012.08.036. Neuron. 2012. PMID: 23040810 Free PMC article. Review. - Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 GAP junctions.
Allison DW, Wilcox RS, Ellefsen KL, Askew CE, Hansen DM, Wilcox JD, Sandoval SS, Eggett DL, Yanagawa Y, Steffensen SC. Allison DW, et al. Synapse. 2011 Aug;65(8):804-13. doi: 10.1002/syn.20907. Epub 2011 Apr 7. Synapse. 2011. PMID: 21218452 Free PMC article. - Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self-administration or conditioned place preference in rats.
Steidl S, Wang H, Wise RA. Steidl S, et al. PLoS One. 2014 Jan 21;9(1):e84412. doi: 10.1371/journal.pone.0084412. eCollection 2014. PLoS One. 2014. PMID: 24465410 Free PMC article. - Differential cholinergic innervation of lemniscal versus non-lemniscal regions of the inferior colliculus.
Noftz WA, Echols EE, Beebe NL, Mellott JG, Schofield BR. Noftz WA, et al. J Chem Neuroanat. 2024 Sep;139:102443. doi: 10.1016/j.jchemneu.2024.102443. Epub 2024 Jun 22. J Chem Neuroanat. 2024. PMID: 38914378
References
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. - PubMed
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. - PubMed
- Bolam JP, Francis CM, Henderson Z. Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience. 1991;41:483–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous